Basic Cupric Carbonate
Basic Cupric Carbonate
Specifications
| Item | Index |
| Appearance | peafowl green particle powder |
| (Cu)%≥ | 55 |
| (Pb)PPM≤ | 5 |
| (Cl)PPM≤ | 10 |
| (Ca)PPM≤ | 10 |
| (Cr)PPM≤ | 5 |
| (Na)PPM≤ | 10 |
| (As)PPM≤ | 5 |
| (Fe)PPM≤ | 10 |
| insoluble matter in hydrochloric acid%≤ | 0.003 |
| moisture %≤ | 2 |
Packing& Storage
| Packing | tons or 25KG, in seal. | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
1.1 Chemical & Physical Properties
| Common Names | carbonic acid, copper salt | Copper(II) carbonate | ||||||
| Structure | 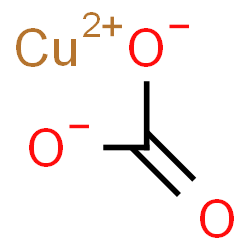 |
||||||
| CAS No. | 7492-68-4 | Boiling Point (℃) | N/A | ||||
| Molecular Weight | 123.555 | Melting Point (℃) | N/A | ||||
| Appearance | N/A | Vapor Specific Gravity | N/A | ||||
| HS Code | N/A | Flash Point (℃) | N/A | ||||
| Solubility | N/A | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | N/A | |
| RIDADR | N/A | |
| WGK Germany | N/A | |
| Packaging Group | N/A | |
| Hazard Class | N/A | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
N/A
What is Basic Cupric Carbonate?
Basic cupric carbonate, also known as basic copper carbonate or copper(II) carbonate hydroxide, is a chemical compound with the formula Cu2(OH)2CO3. It is an inorganic compound that occurs as a greenish-blue solid.
Basic cupric carbonate is commonly prepared by mixing solutions of copper(II) salts, such as copper sulfate or copper nitrate, with solutions of alkali metal carbonates or bicarbonates. The reaction between the copper salt and carbonate or bicarbonate ions leads to the precipitation of basic cupric carbonate.
The compound has a layered structure and exists as a hydrated form, known as malachite, in nature. Malachite is a common mineral and is valued for its vivid green color. It has been used as a pigment and gemstone for centuries.
Basic cupric carbonate is also used in various industrial applications. It is utilized as a pigment in ceramics, paints, and coatings, providing a green color. It can also be employed as a fungicide and algicide in agriculture and horticulture. In addition, the compound has applications in the synthesis of other copper compounds and as a catalyst in certain chemical reactions.
