Cupric Chloride
Specifications
| Cupric Chloride | Export High-purit | A.R. | C.P. | USP31 | Refined grade | Technical first grade | Technical second grade |
| Item | Spec. | Spec. | Spec. | Spec. | Spec. | Spec. | Spec. |
| Assay(as CuCl2.2H2O))% | ≥99.0 | ≥99.0 | ≥99.0 | 99.0-100.5 | ≥98.5 | ≥98.0 | ≥97.0 |
| Water insoluble% | ≤0.003 | ≤0.005 | ≤0.02 | ≤0.01 | ≤0.02 | ≤0.02 | ≤0.02 |
| Loss on drying % | —— | —— | —— | 20.9-21.4 | —— | —— | —— |
| Nitrates(NO3)% | —— | ≤0.01 | ≤0.03 | —— | —— | —— | —— |
| Sulphates(SO4-)% | ≤0.01 | ≤0.003 | ≤0.01 | ≤0.005 | ≤0.02 | ≤0.03 | ≤0.04 |
| Iron(Fe)% | ≤0.002 | ≤0.002 | ≤0.002 | ≤0.005 | ≤0.005 | ≤0.02 | ≤0.02 |
| Arsenic(As)% | ≤0.01 | ≤0.0002 | ≤0.0005 | —— | —— | —— | —— |
| Non-precipitate in sulfureted hydrogen(As sulphates) % | —— | ≤0.05 | ≤0.2 | —— | —— | —— | —— |
| Zinc(Zn)% | —— | —— | —— | —— | ≤0.02 | ≤0.02 | ≤0.03 |
| Lead(Pb) | ≤0.002 | —— | —— | —— | —— | —— | —— |
| Nitrate(N) % | ≤0.004 | —— | —— | —— | —— | —— | —— |
| Potassium(K)% | —— | —— | —— | ≤0.01 | —— | —— | —— |
| Calcium(Ca)% | ≤0.01 | —— | —— | ≤0.005 | —— | —— | —— |
| Nickel(Ni)% | ≤0.001 | —— | —— | ≤0.01 | —— | —— | —— |
| Sodium(Na)% | ≤0.005 | —— | —— | ≤0.02 | —— | —— | —— |
| Organic volatiles | —— | —— | —— | —— | —— | —— | —— |
| Barium(Ba)% | ≤0.05 | —— | —— | Pass | —— | —— | —— |
| PH(5 % solution) | 3.0-3.8 | —— | —— | —— | —— | —— | —— |
Packing& Storage
| Packing | 25kg woven bag with double PE liners | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
| Common Names | Cupric Chloride | Copper(II) chloride dihydrate | dichlorocopper,dihydrate | ||||||
| Structure | 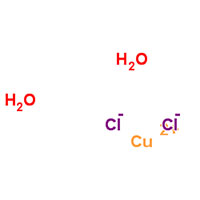 |
||||||
| CAS No. | 10125-13-0 | Boiling Point (℃) | 993ºC | ||||
| Molecular Weight | 170.483 | Melting Point (℃) | 100 °C (dec.)(lit.) | ||||
| Appearance | Blue-green orthorhombic crystals | Vapor Specific Gravity | 2.54 | ||||
| HS Code | 2827399000 | Flash Point (℃) | N/A | ||||
| Solubility | Soluble in alcohol and ammonia, acetone | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | S26-S60-S61-S36 | |
| RIDADR | UN 2802 8/PG 3 | |
| WGK Germany | 3 | |
| Packaging Group | III | |
| Hazard Class | 8 | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
1. Hydrochloric acid method A certain amount of copper oxide is gradually added to the reactor containing hydrochloric acid, and the acidolysis reaction is added while stirring to generate copper chloride. When the pH value of the reaction solution is 2 and the concentration is 35~37 °Bé, the reaction is completed, and sodium hypochlorite is added to the clear solution after standing clarification, so that ferric iron is oxidized to ferric iron, and hydrolysis filtration is removed. The filtrate is concentrated by evaporation, cooled and crystallized, centrifuged, and dried at 60~70 °C to obtain a finished copper chloride product, and its reaction formula is as follows:
Cuo+2HclCucl2+H2O2.A mixed solution of copper added to water, hydrochloric acid and nitric acid. After the reaction is completed, filtration, evaporation, cooling, suction and filtration of crystals and re-dissolution in water, and then evaporation until a crystalline film appears, and then cooling, crystallization, alcohol washing, drying, pure copper chloride. 3. Heat 1 part copper and 5 parts 25% hydrochloric acid in a beaker according to the mass ratio, and slowly add 2.7 parts of 25% nitric acid to completely dissolve the metal copper, filter the solution and evaporate it on a water bath, and cool when the crystallization begins to precipitate on the liquid surface. The crystals are sucked through a glass filter and then recrystallized in boiling ethanol containing several drops of hydrochloric acid. The resulting crystals are rapidly dried and stored. 4. Soak the slender strip of electrolytic copper in 20% hydrochloric acid, wash it with distilled water and dry it for later use. Add 4 kg of analysis to 10 kg of washed copper strips
Pure hydrochloric acid and 30kg of distilled water, then add about 0.4kg of copper chloride mother liquor, heat to 60 °C, and pass industrial chlorine gas under the liquid surface: 10125-13-0 preparation After the reaction for 24h, the reaction solution is heated to 70 °C, and then the industrial chlorine gas is passed under the liquid surface for reaction: 10125-13-0 preparation Until the solution is transparent emerald green, stop the reaction. The solution is filtered, the filtrate is evaporated and concentrated until large bubbles appear, the heating is stopped, the crystallization is cooled to 15 °C, and after no more crystallization is precipitated, the crystals are centrifuged and dried, spread in a glass dish, and baked in the oven at 60 °C to semi-dry,
Non-stick scoop is fine.
5. Add fine copper wire (or small copper sheet) in aqua regia to carry out the reaction ( intense, slow down, heating at the end): 10125-13-0 After the reaction, filter the solution. When the filtrate is evaporated and concentrated to 1/3 of the original volume, it is cooled and crystallized, and then re-dissolved in water after filtration, heated and evaporated until a crystalline film appears, filtered dry, washed with ethanol and spread on a glass plate, first dried at 30 °C and intermittent stirring, when the green turns to blue-green, the temperature is raised to 50 °C for drying, that is, pure grade copper chloride hydrate can be analyzed.
