Potassium Bisulphate
Potassium Bisulphate(potassium acid sulfate )
Specifications
| Potassium Bisulphate | A.R. | C.P. | Catalyst grade | Technical grade |
| Item | Index | Index | Index | Index |
| Assay % | 99~101 | 99.0-101.0% | ≥99.9 | ≥99.5 |
| Clarity test | PASS | PASS | —— | —— |
| Water insoluble% | ≤0.002 | ≤0.005 | ≤0.05 | ≤0.2 |
| Chlorides(Cl) % | ≤0.0005 | ≤0.002 | —— | —— |
| Nitrogen compound(N) % | ≤0.002 | ≤0.005 | ≤0.01 | —— |
| Phosphate(PO4)% | ≤0.001 | ≤0.002 | ≤0.002 | —— |
| Silicates(SiO3) % | ≤0.001 | ≤0.005 | ≤0.005 | —— |
| Na% | ≤0.02 | ≤0.05 | ≤0.05 | —— |
| Mg % | ≤0.0005 | ≤0.002 | ≤0.002 | —— |
| Al % | ≤0.001 | ≤0.005 | ≤0.005 | —— |
| Ca% | ≤0.002 | ≤0.005 | ≤0.005 | —— |
| Fe % | ≤0.0005 | ≤0.002 | ≤0.002 | —— |
| As % | ≤0.0003 | ≤0.0005 | ≤0.0005 | —— |
| Heavy metal(as Pb) % | ≤0.0005 | ≤0.002 | ≤0.002 | —— |
Packing& Storage
| Packing | In 25Kg/woven bag lined with double-layer plastic bag | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
1.1 Chemical & Physical Properties
| Common Names | Potassium bisulfate | potassium hydrogen sulfate |
||||||
| Structure | 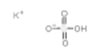 |
||||||
| CAS No. | 7646-93-7 | Boiling Point (℃) | 330ºC at 760 mmHg | ||||
| Molecular Weight | 136.16900 | Melting Point (℃) | 214 °C(lit.) | ||||
| Appearance | White flake or granular crystals | Vapor Specific Gravity | N/A | ||||
| HS Code | 2833299090 | Flash Point (℃) | N/A | ||||
| Solubility | Soluble in water, the solution is strongly acidic and insoluble in ethanol. | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | S26-S36/37/39-S45 | |
| RIDADR | UN 2509 8/PG 2 | |
| WGK Germany | 1 | |
| Packaging Group | II | |
| Hazard Class | 8 | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
1. Mix 87g of K2SO4 with 27mL of concentrated H2SO4 (relative density 1.84) in a platinum dish and carefully heat until the reactants become completely transparent. The molten body of the reactant is poured into a porcelain or platinum container cooled in cold water, and after the material is solidified, it is mashed and transferred to a closed bottle for storage.
2. Dissolve 50kg of industrial potassium carbonate with 40kg of distilled water, stir while heating, and filter out insoluble impurities with three-layer filter paper after dissolution. Slowly add industrial sulfuric acid to the clear filtrate under stirring, carry out the reaction, when the solution pH is 5~6, stop adding acid and stand. After the precipitation is complete, filtered, centrifuged, and washed with distilled water until the ion content of the calf is qualified. Add an appropriate amount of distilled water according to the amount of 7.5kg of water per 10kf of potassium sulfate, slowly add analytical pure sulfuric acid (9kg sulfuric acid per 10kg of potassium sulfate) under stirring, steam heating to dissolve all of them, add a little washed activated carbon, heat and stir for a while, and filter while hot under reduced pressure. The clear and transparent filtrate is heated and evaporated at 80~100 °C (to prevent boiling during evaporation), stop heating when the density is 1.65~1.68, naturally cool the crystallization, and when the crystallization is complete (about 25~30 °C), take out and spin dry, and dry in the oven at 100 °C.
