Sodium Carbonate Decahydrate
Specifications
| Sodium Carbonate Decahydrate | Reagent grade A.R. | Reagent grade C.P. | Specific grade |
| Item | Index | Index | Index |
| Assay(Na2CO3.10H2O)% | ≥99.0 | ≥99.0 | ≥99.0 |
| Clarity test | Pass | Pass | Pass |
| Water insoluble% | ≤0.005 | ≤0.01 | ≤0.02 |
| Chlorides % | ≤0.001 | ≤0.003 | ≤0.005 |
| Sulfur compound(SO4)% | ≤0.003 | ≤0.005 | ≤0.01 |
| Phosphate(PO4)% | ≤0.0005 | ≤0.002 | ≤0.005 |
| Total nitrogen(N) % | ≤0.0005 | ≤0.001 | ≤0.005 |
| Silicates(SiO3)% | ≤0.001 | ≤0.005 | ≤0.01 |
| Mg% | ≤0.0003 | ≤0.002 | ≤0.005 |
| Al% | ≤0.0005 | ≤0.003 | ≤0.005 |
| K% | ≤0.003 | ≤0.01 | ≤0.02 |
| Ca% | ≤0.003 | ≤0.008 | ≤0.01 |
| Fe% | ≤0.0002 | ≤0.0003 | ≤0.001 |
Packing& Storage
| Packing | In 25Kg/woven bag lined with double-layer plastic bag | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
1.1 Chemical & Physical Properties
| Common Names | Sodium Carbonate Decahydrate | Crystal soda | ||||||
| Structure | 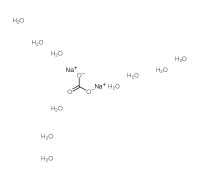 |
||||||
| CAS No. | 6132-02-1 | Boiling Point (℃) | 445.021ºC at 760 mmHg | ||||
| Molecular Weight | 286.14100 | Melting Point (℃) | 34 °C | ||||
| Appearance | Colorless transparent crystal, easy to weathering in the air. | Vapor Specific Gravity | N/A | ||||
| HS Code | Flash Point (℃) | 222.941ºC | |||||
| Solubility | Dissolved in 2 parts cold water, 0.25 parts boiling water. Soluble in glycerol, insoluble in ethanol | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | S22-S26 | |
| RIDADR | 3262 | |
| WGK Germany | 1 | |
| Packaging Group | III | |
| Hazard Class | N/A | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
1. Add three times the amount of distilled water to the industrial sodium carbonate, heat, stir to dissolve, add an appropriate amount of sodium sulfide according to the total amount of Pb2+, Cu2+, As2O3 contained in the raw materials, stir thoroughly, stand, and filter after PbS, CuS, As2O3 is completely precipitated. In the clear filtrate, add the total volume of 1% hydrogen peroxide and 0.1% activated carbon, and add sodium oxalate according to the Ca2+ seed content in the raw material, stir well, heat to boiling, and filter while hot. The clear filtrate is evaporated and concentrated until the crystalline film is formed, the heating is stopped, the crystallization is cooled, and the centrifugation is centrifuged to obtain the analysis of pure crystalline sodium carbonate. To prepare high-grade pure crystalline sodium carbonate, only the above finished product needs to be recrystallized once.
