Trimethyl Phosphate: A Grounded Look at Its Past, Properties, Applications, and Future
Historical Development
Back in the early 20th century, chemists started to pay closer attention to organophosphates, partly driven by the search for new solvents and industrial chemicals that could nudge technology forward. Trimethyl phosphate came onto the scene as researchers looked for robust methylating agents and nonflammable solvents. Military needs in World War II, as well as broader industrial expansion, increased demand for such specialty chemicals. Laboratories started refining its synthesis, improving both yield and purity. Over the years, knowledge spread from defense and industrial contexts into academic circles, where the focus shifted toward its underlying chemistry and safety. Each stage brought changes—not just to how production was scaled up, but how society regulated its use and disposal.
Product Overview
Trimethyl phosphate shows up in industry as a colorless, slightly viscous liquid with a mild odor. Its main selling points include good solvency, low volatility, and nonflammability; it’s not the flashiest compound, but engineers rely on it when they need stable, reliable performance under specific conditions. Compared to other organophosphates, TMAP offers a lower risk of accidental ignition, which brings peace of mind to those overseeing manufacturing or large-scale storage. It’s also less physically hazardous compared to the heavier or more reactive phosphate esters, making it a frequent choice in operations where chemical steadiness matters as much as effectiveness.
Physical & Chemical Properties
Trimethyl phosphate consists of a central phosphorus atom bonded to three methoxy groups and a double-bonded oxygen. At room temperature, it forms a clear, mobile liquid that handles well, with a boiling point around 197°C and a melting point that hovers near −46°C. Its moderate polarity allows it to dissolve both polar and some non-polar compounds. The liquid absorbs moisture from the air, but doesn't react violently with water. Chemically, TMAP behaves in a stable, predictable way, resisting oxidation under most laboratory conditions. Still, acids and bases can split its ester bonds, and it transmutes steadily under high temperatures, where decomposition releases toxic fumes, including phosphoric acid and methyl ethers. Chemists appreciate its high dielectric constant compared to many ethers, which enables specific applications in batteries and other electronic settings, even though handling always calls for respect due to its toxicity.
Technical Specifications & Labeling
Manufacturers usually grade trimethyl phosphate according to purity, water content, and appearance. Industrial and laboratory batches target purity above 99 percent. Reputable suppliers offer precise certificates of analysis for each batch, listing levels of impurities like methanol and dimethyl phosphate, plus clarity and color indexes. Labels must show its UN number (2328), warning icons for toxicity and environmental hazard, advice for storage in tight containers, and temperature guidelines to guard against unwanted degradation. End users rely on this technical information—not only for compliance, but for everyday safety and process optimization.
Preparation Method
Labs and chemical plants typically produce TMAP by reacting phosphorus oxychloride (POCl3) with methanol under controlled alkaline conditions, usually using a base to neutralize the hydrogen chloride byproduct. Process engineers monitor temperature and pH closely, since the exothermic reaction requires careful handling to prevent side reactions or yield drops. Over the years, researchers have fiddled with catalysts and alternative routes to minimize waste and reduce exposure to chlorinated intermediates, but the basic recipe has held steady. Improvements in purification—especially repeated distillation under reduced pressure—give companies a product that meets the high standards of specialty chemical buyers. Small-scale synthesis sticks to laboratory glassware, but bulk operations employ corrosion-resistant reactors and continuous handling to push efficiency and cut costs.
Chemical Reactions & Modifications
In practice, trimethyl phosphate acts as a methylating agent, yielding methyl esters from various nucleophiles under the right catalytic conditions. Oxidizing environments don’t bother it much, but strong acids or bases chew through its molecular structure and throw off methyl alcohol and other byproducts. Graduate students learn the dangers of combining TMAP with halides and high heat, as pressure can build quickly and dangerous gases emerge. Many downstream uses hinge on modifying this compound—sometimes hydrolyzing it to diphosphate esters, other times using it as a stepping stone to more tailored organophosphorus agents for fire retardant formulations. Avoiding unintended side reactions, especially with amines or strong acids, keeps process chemists on their toes.
Synonyms & Product Names
This compound travels through catalogs under several names: trimethyl orthophosphate, phosphoric acid trimethyl ester, TMP, and methyl phosphate. In international trade, its CAS number—512-56-1—serves as a principal identifier. Some regional suppliers pitch it under proprietary trade names, but law requires clear documentation and specification cross-referencing. Multiple names can trip up new buyers, but the consistency of regulatory labeling diminishes confusion across markets.
Safety & Operational Standards
Anyone working with trimethyl phosphate faces health risks if they don’t observe the right precautions. Toxicity mainly comes in through inhalation or skin contact, irritating eyes and respiratory tracts—and potentially putting nervous systems at risk with chronic exposure. OSHA and international safety authorities require handling with gloves, goggles, and local exhaust ventilation. Spill cleanup demands absorbent media and strict waste containment, since runoffs threaten aquatic life. Safety data sheets supply in-depth hazard guidance. In my own experience, regular air monitoring and proper medical surveillance matter as much as the material safeguards. Regulatory compliance gets backed up by routine drills and audits, which help reduce accidents and provide clarity during emergencies.
Application Area
Trimethyl phosphate finds itself in a tight set of industries, but the impacts ripple out widely. Most often, the chemical serves as a methylating agent in organic syntheses for pharmaceuticals and fine chemicals. It also slides into electrolytes for lithium-ion battery research, owing to its high dielectric strength. Add it to flame retardants, and building materials stand up better to ignition hazards. Labs reach for it as a solvent in spectroscopy and chromatography, because it dissolves tricky analytes where other solvents stall. Aerospace and polymer manufacturers sometimes leverage its stability for specialty resins and lubricants. While not as widespread as everyday solvents like acetone, its niche value remains strong and entrenched.
Research & Development
Research priorities swing between making TMAP production safer and more sustainable, and pushing new applications in tech-heavy sectors. Active efforts target greener methods that reduce halogenated wastes and cut down on energy-intensive distillations. Electrochemical researchers are trying to adapt TMAP into novel battery chemistries by tweaking its interaction with lithium salts. Health scientists, meanwhile, refine testing methods to track residue and exposure routes, aiming to bridge gaps between laboratory research, industrial usage, and public health. Collaborations between university chemists and manufacturing engineers often yield new derivatives or hybrids that carry the utility of TMAP into rarefield corners of medicine and energy storage. Regulatory compliance also shapes R&D, as companies track shifting legal standards and public concerns over chemical safety.
Toxicity Research
Toxicologists classify trimethyl phosphate as moderately harmful—acute effects tend toward irritation, but higher doses linger and mount toward nerve damage. Chronic exposure now ranks as a particular focus after outbreaks of nerve damage in industrial settings—something that hits home in countries with less rigorous oversight. Test results in animals have pointed to organ damage and delayed metabolic breakdown, which push researchers to tighten exposure limits and push for better air handling in factories. Current research tracks breakdown products in the environment, as wastewater streams sometimes carry low levels of TMAP into surface water and soils. Epidemiologists follow up with factory workers, cross-matching health reports with ambient air and biological monitoring data. While fatalities are rare, the push for safer substitutes and improved PPE is gaining ground across the sector.
Future Prospects
Even as stricter regulations and new green chemistry initiatives shift industrial focus, trimethyl phosphate isn’t likely to fade away anytime soon. Demand for specialty solvents, lithium battery electrolytes, and flame retardants continues to rise across Asia and North America. Advances in life sciences, electronics, and energy often spin off new needs for versatile phosphates. Researchers keep hunting for biodegradable or less-toxic replacements, but so far, few alternatives offer the same blend of stability and performance for specialized applications. Shifts in public opinion could increase pressure to phase out high-toxicity compounds, yet the technical advantages of TMAP keep it in the pocket for scientists and engineers who care about precision and reliability. As long as safer best practices, technological improvements, and transparent research guide its use, TMAP will keep playing a role in both legacy industries and next-generation technologies.
How Trimethyl Phosphate Fits into Modern Industry
Trimethyl phosphate comes up in quite a few places, especially where chemistry meets manufacturing. I first ran across it when I worked around laboratories focused on new materials. Recognizing chemicals beyond their formulas helps to put their roles in context. Trimethyl phosphate is one with more reach than its name suggests.
Factories use this compound often as a solvent. Its properties give it an edge where sturdy, stable reactions are needed. Instead of water, which can cause some chemicals to break down or react too quickly, trimethyl phosphate steps in. For example, folks making coatings or plastics twist chemical chains together in ways that must avoid side reactions. This phosphate brings a certain calm because it won’t set off unexpected sparks in the mix. The demand for safer solvents rises each year, and trimethyl phosphate often replaces riskier, more toxic options from decades past.
Supporting Electronics and Pharmaceuticals
People rarely think about the inner workings of electronics, though every new phone or laptop relies on layers of fine-tuned chemicals. Trimethyl phosphate supports the creation of these micro-thin coatings on circuit boards. Strong enough to break down certain minerals but gentle enough not to damage tiny wires, this compound manages a balancing act that keeps electronics both safe and dependable. I saw this firsthand while visiting a semiconductor plant; the way workers treat chemical handling like an art form underscores just how much depends on getting it right.
Pharmaceuticals depend on purity, and manufacturers look for chemicals that won’t add unexpected impurities. Trimethyl phosphate acts as a reagent during manufacturing, especially with certain antibiotics and specialty medicines. Quality here means health and safety down the line. Even small quantities can change the outcome, so factories test their batches with impressive vigilance. Researchers point to its predictable reactions as a reason for choosing it over older, less reliable chemicals.
Fire Safety and Flame Retardants
Household products and building materials get safer with the addition of trimethyl phosphate. It’s part of the additive mixes that keep items from catching fire too easily. Mats, foams, and plastics for cars and furniture include these ingredients, providing minutes of precious time in emergencies. Stories of fires spreading slowly thanks to flame retardants highlight the value of materials science in real life. Trimethyl phosphate itself doesn’t burn easily, so it becomes a helpful shield in environments full of electrical gear or heated surfaces.
Protecting People and Environment
Safety always raises questions with chemical compounds. Regulation keeps a close eye on trimethyl phosphate, pushing manufacturers to minimize exposure and train staff well. The EPA and other agencies keep tabs on its handling, calling for strict procedures in both transportation and disposal. Workplace air monitors and protective gear shield folks from risks. Responsible companies share their safety data so communities know what’s nearby and how it’s being managed.
Scientists explore potential bio-based alternatives for each use, aiming for even safer and cleaner processes. Trimethyl phosphate earned its place through performance and reliability, but new options may take root as technology changes what’s possible. Encouraging innovation here means more than environmental checklists—it brings long-term security across industries balancing production with protection.
Understanding What’s on the Label
Trimethyl phosphate, often found in labs, industrial applications, and occasionally in research settings, deserves way more attention than it often gets. The name alone sounds complicated enough to scare most people away from reading the label twice. Yet, the real concern pops up when considering what this chemical can do if handled rashly. Common sense tells us that strong-smelling laboratory chemicals rarely belong anywhere near unprotected skin or open containers. I’ve seen too many scientists ignore the warnings, thinking gloves and goggles offer some sort of magic shield. Of course, chemicals still have a way of slipping through the smallest cracks in protocol.
What the Science Shows So Far
Ask toxicologists about trimethyl phosphate, and a clear pattern emerges. The facts come from decades of animal testing and worker experience. Fact: it irritates the skin, eyes, and even lungs if vapors drift up your nose. Short doses in the lab can trigger coughing and discomfort, while repeated exposure can lead to chronic health issues like respiratory trouble and potential damage to internal organs. The European Chemicals Agency lists it as harmful if swallowed or inhaled and not exactly a friend to aquatic life either. NIOSH points out that prolonged skin contact can cause redness or more severe burns, so short sleeves and open shoes simply won’t cut it. It isn’t only about what happens in the lab—improper disposal raises environmental risks for people living nearby.
From Headlines to Everyday Experience
No one likes headlines about unexplained headaches and rashes at work. Still, this sort of news pops up every so often in industries where personal protective equipment sometimes falls by the wayside. I recall working in an older facility where chemical fumes made lunch breaks downright miserable. Eyes burned, throats itched, and a few coworkers developed allergies that never faded away completely. Once, poorly maintained ventilation almost forced an evacuation. Most folks in heavy industry or science walk a fine line between safety and efficiency. But all it takes is one leak, one spill, or one overlooked safety drill to highlight why safe handling matters.
What Makes Trimethyl Phosphate Dangerous?
Trimethyl phosphate can move through the skin, and its vapors seep into the lungs. Acute exposure, like splashing or inhaling a concentrated vapor, doesn’t end with mild symptoms. It can cause headaches, breathing trouble, or, after high exposure, more severe systemic issues. The trickiest part comes from how easily people underestimate it. Invisible vapors and delayed symptoms are a recipe for long-term problems if no one speaks up. The U.S. EPA also tags it as a possible carcinogen, raising questions about occupational cancer risk in factories and research spaces.
Protecting Health with Practical Steps
Reliable, science-backed protection starts with proper engineering controls. Strong fume hoods, monitored storage, and tough gloves cut down on nearly all known risks. In my experience, most accidents stem from rushed jobs or neglected maintenance. Nobody wins when shortcuts lead to a trip to the hospital. Safety data sheets aren’t just paperwork—they tell the story of hundreds of studies and real-world incidents. The most grounded approach always involves reading the label, asking questions, and following established procedures. Replacing trimethyl phosphate with safer alternatives, wherever possible, gives workers the best shot at staying healthy in the long run.
Why Trimethyl Phosphate Storage Isn’t Just Routine
Trimethyl phosphate comes with real risks. This chemical serves in manufacturing, research, and sometimes as a solvent. It carries hazards including toxicity and the danger of releasing noxious fumes when exposed to high temperatures. My early days in a laboratory taught me just how quickly mistakes can escalate in a cramped storage room. Every chemical storage rule exists for a reason, and ignoring even one can cause serious trouble.
Setting the Right Environment
Temperature and humidity stand out as main factors. Trimethyl phosphate keeps best in a cool, dry spot, away from heat sources. Many labs use dedicated cabinets with temperature control because ambient heat increases evaporation and raises inhalation risks if vapors escape. A neighbor once left a bottle near a sunny window, which warped the cap and caused a slow leak—alert noses noticed in time, but no one wants that close call. Choose locations far from direct sunlight, radiators, and vents that blow warm air.
Separation Means Safety
This isn't a chemical to mix with others without checking a compatibility chart. Store trimethyl phosphate well apart from oxidizers, strong acids, or bases. Mixing up bottle placement in a cluttered cabinet sets up a chain reaction that firefighters see all too often. Dedicated shelving, labeled with clear, simple signage, keeps confusion to a minimum.
Containment, Containers, and Labels
Original containers design matters more than some realize. Glass or high-quality plastic, with caps that fit tight, blocks slow leaks or absorption through the bottle walls. Double containment—a sealed outer vessel—adds backup protection, especially in older buildings without modern spill-control infrastructure. Proper labeling saves lives. Every side of a storage container should give clear hazard information and date of receipt, helping you spot expired stock before it causes trouble. One expired bottle sometimes survives cabinet cleanouts, leading to accidents later.
Ventilation and Protective Gear: Not Optional
Sometimes people get lax about fume hoods or ventilation. If a bottle must open—even briefly—work within a properly maintained fume hood. Inhalation delivers this chemical right into lungs, and I’ve seen colleagues complain of burning throats after one careless exposure. Eye and hand protection must be standard. Splash goggles and good nitrile gloves cost little compared to a night at the ER.
Disposal and Emergency Response
Too often, disposal gets only a footnote. Local regulations set chemical disposal standards for a reason. Pouring trimethyl phosphate down a drain can damage wastewater systems and harm water quality. Instead, partner with accredited waste management firms, follow their instructions, and keep clear records. Know right away where spill kits sit and run regular drills for chemical spill response—muscle memory beats panic every time. In one incident in my early career, a quick-thinking tech used the right absorbent pads and prevented a small spill from reaching the lab floor drains, saving a lot of headaches and paperwork.
Sharpening Culture and Oversight
Training programs do more than tick regulatory boxes. A culture where questions are welcome, and regular refreshers keep everyone alert, prevents mistakes. Encourage team members to speak up about any broken seals, expired stock, or labeling issues. Oversight by supervisors must include regular spot checks. Fewer incidents come from environments where no one cuts corners—even on a hectic day.
Looking ahead, new storage technologies and better monitoring sensors offer hope for lower-risk workspaces. No safeguard replaces vigilance or a culture of mutual accountability. Trimethyl phosphate doesn’t forgive carelessness. Following these steps protects both people and property every single shift.
A Look at Trimethyl Phosphate’s Identity
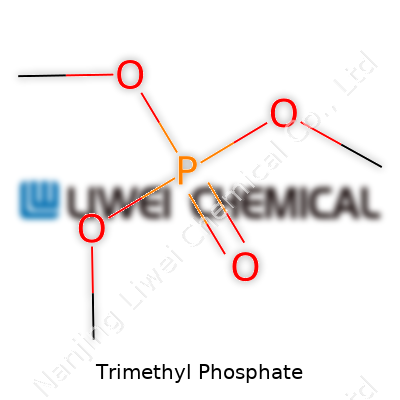
Trimethyl phosphate—a colorless, flammable liquid with a subtle, pleasant odor—shows up in labs and industrial sites far more than most realize. Its chemical formula, C3H9O4P, sketches out a picture of three methyl groups joined to a single phosphate. The molecule falls into the family of organophosphates, which stretch from everyday flame retardants to nerve agents. Scrutinizing its structure, you find a central phosphorus atom double-bonded to an oxygen atom, surrounded by three methoxy groups. Chemists draw its structure as (CH3O)3PO, and in three dimensions, the shared oxygen bridges tip it into a trigonal pyramidal shape.
The Real-World Uses
Anyone who has worked around chemical synthesis or spent time in analytical labs runs into trimethyl phosphate eventually. I saw it used most often in methylation reactions—helping add methyl groups to organic compounds as part of pharmaceutical research. In that role, it helps chemists tweak drugs, dyes, and surfactants to change how they behave in living systems. Manufacturers lean on it as a solvent, benefiting from its ability to mix with water and alcohols. Production of flame retardants and plasticizers counts on it, especially for products exposed to high temperatures.
Health and Safety Risks
Handling trimethyl phosphate brings its own hazards. Even brief exposure can irritate eyes and respiratory tracts. Safety datasheets warn of possible central nervous system effects after long-term, high-dose contact. Inhalation and skin contact need to be taken seriously—so gloves, goggles, and ventilation become non-negotiable. Thankfully, it’s far less toxic than organophosphates used as pesticides, but that doesn’t make it harmless. As I learned in chemical safety courses, any spill clean-up starts with containment and ventilation, followed by storing waste in appropriate containers for specialized disposal. Rinsing with water helps only in small, controlled cases; professional practice always treats it with respect.
Environmental Considerations
Manufacturers and research outfits face scrutiny over organophosphates, including trimethyl phosphate, because of their persistence in water systems. Runoff from production facilities can slip into local waterways, where it may disrupt aquatic life. Regulatory guidelines restrict emissions and mandate reporting above specific thresholds. Labs and plants must invest in containment, treatment, and monitoring protocols. Having seen community concerns over chemical plants rise over the past decade, transparency and compliance shape both public trust and business longevity.
Improving Practices
Progress boils down to common-sense steps and smart innovation. Chemists continue hunting for greener alternatives and refine ways to recover or recycle trimethyl phosphate from waste streams. Educators emphasize precise measurement and disposal, which cuts down on unnecessary waste and accidents. From my own experience in lab management, regular training and well-maintained safety gear do more than keep accidents at bay—they create a culture where everyone goes home safe. Investments in closed-system technology for industrial users—where no fumes escape—can drop exposure risks close to zero.
The Chemistry in Context
Appreciating trimethyl phosphate means understanding the balance it strikes between useful chemistry and potential harm. A deceptively simple formula turns out to drive crucial synthesis steps, with far-reaching applications in science and manufacturing. Keeping its uses safe and sustainable demands diligence, up-to-date knowledge, and ongoing commitment to environmental stewardship. Every bottle stored on a shelf comes with a responsibility as real as any lab experiment.
Understanding the Risk
Trimethyl phosphate isn’t a household name, but those who work with chemicals know its power and potential hazards. Used in the production of flame retardants, gasoline additives, and even as a solvent, this clear liquid comes with serious baggage: it harms the nervous system, irritates skin and eyes, and may cause harm if inhaled or ingested. That’s a real risk for anyone working in labs, factories, or handling transportation and storage.
Right Response Starts with Preparedness
No one wants to scramble when a spill happens. I remember my days working at a small research facility—our protocols saved us from chaos more than once. People can’t just trust good luck: companies that use or transport trimethyl phosphate owe it to everyone in the building to have a plan. Spill kits close to work areas, safety data sheets within arm’s reach, and clear instructions posted where people can see them—that’s the difference between a headache and a disaster.
OSHA lays out specific requirements for chemical handling and emergencies. Smart organizations don’t see these as boxes to check; they treat them as living guides. Regular drills build confidence. Staff learn how to use absorbents, wear the right gloves and respirators, and get the area cleared before real harm happens.
The Spill Happens—What Next?
The knee-jerk reaction to “just mop it up” doesn’t fly with trimethyl phosphate. Small spills might look harmless but still pose a health threat. Ventilation is the first step. Open windows, crank up the fans, get fresh air moving. Next, cordon off the area. I’ve seen folks use caution tape, traffic cones, even heavy boxes—anything to keep unprotected people out.
Personal protective equipment matters. Chemical-resistant gloves, safety goggles, a decent respirator—not everyone keeps these at home, but every company that stocks dangerous substances should have them on hand. Absorbent materials made for chemical spills work far better than the average paper towel. Once the liquid’s contained, scoop it up with non-sparking tools, and seal it in labeled containers. This part sounds simple on paper but takes real focus to avoid splashing or spreading contamination.
After the Clean-Up: Health Takes Priority
No one should “tough it out” if they get exposed. Trimethyl phosphate isn’t just a skin irritant—it can cause real damage. Medical evaluation shouldn’t be up for debate. Any symptoms like headache, nausea, or dizziness mean it’s time to seek attention. Employers who invest in first-aid training and have numbers for emergency clinics posted get people back on their feet quicker.
Long-Term Solutions and Prevention
Designing good ventilation systems, setting up storage in well-marked, ventilated areas, and using secondary containment all go a long way. Regular maintenance keeps leaks to a minimum. Training isn’t just a one-time event—new hires and seasoned workers alike benefit from refresher courses. Equipment updates, better signage, and using less hazardous alternatives where possible should always stay on the table.
Spill management doesn’t just keep workers safe—it protects groundwater, public health, and even the company’s reputation. Putting people and safety first pays off every day, not just when something goes wrong.
| Names | |
| Preferred IUPAC name | Trimethoxyphosphane |
| Other names |
Phosphoric acid trimethyl ester
TMP Trimethylphosphoric acid Methyl phosphate Methylphosphoric acid trimethyl ester |
| Pronunciation | /traɪˈmɛθɪl fəʊˈsfeɪt/ |
| Identifiers | |
| CAS Number | 126-79-4 |
| Beilstein Reference | 1465078 |
| ChEBI | CHEBI:4786 |
| ChEMBL | CHEMBL31888 |
| ChemSpider | 6221 |
| DrugBank | DB01855 |
| ECHA InfoCard | 100.005.064 |
| EC Number | 204-603-4 |
| Gmelin Reference | Gmelin Reference: 103778 |
| KEGG | C19322 |
| MeSH | D014251 |
| PubChem CID | 7861 |
| RTECS number | TX9625000 |
| UNII | W84X070U8B |
| UN number | UN1282 |
| Properties | |
| Chemical formula | C3H9O4P |
| Molar mass | 140.073 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.219 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | 0.02 |
| Vapor pressure | 0.49 mmHg (20°C) |
| Acidity (pKa) | 1.0 (at 25 °C) |
| Basicity (pKb) | 'Trimethyl Phosphate' has a basicity (pKb) of "−4.2 |
| Magnetic susceptibility (χ) | -50.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.385 |
| Viscosity | 1.697 cP (20°C) |
| Dipole moment | 3.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 314.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -759.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1807.4 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS06,GHS03 |
| Signal word | Danger |
| Hazard statements | H301, H311, H331, H319, H370 |
| Precautionary statements | P210, P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P311, P330, P337+P313, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0-Health:1 Flammability:2 Instability:0 |
| Flash point | 54 °C |
| Autoignition temperature | 535 °C |
| Lethal dose or concentration | LD50 Oral Rat 2,150 mg/kg |
| LD50 (median dose) | 1,600 mg/kg (rat, oral) |
| NIOSH | WF1575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Trimethyl Phosphate: "3 mg/m³ (TWA) |
| REL (Recommended) | 0.2 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Trimethyl Phosphite
Phosphoric acid Dimethyl phthalate Triethyl phosphate |

