Potassium Bisulphate: Digging Deep Into Its Journey and Impact
Understanding the Origins of Potassium Bisulphate
Potassium bisulphate has a history that crosses the boundary between old-world chemistry and today’s industrial backbone. Back in the 18th century, European chemists stumbled upon this compound while probing minerals and salts. The demand picked up as glassmakers learned it could help purify raw materials by removing pesky iron stains. Through the 19th century, potassium bisulphate worked its way into laboratories and workshops, prized for tasks from analytical chemistry to crown glass production. My own first brush with it came in a university setting, fumbling with heavy flasks and the distinct sting of acid fumes. The hands-on realization? Potassium bisulphate isn’t just a relic—it underpins products and processes we lean on daily, from fertilizers to food processing.
Getting to Know the Product
This salt, often showing up as white or slightly pink granules, goes by names like potassium hydrogen sulfate and monopotassium sulfate. Chemists reach for it because of its convenient acidity and reactivity, using it for pH adjustments or as a drying agent in laboratories. Over the years, manufacturers have refined its purity and particle size, driven by industries needing tighter specifications. So, buying a bag off the shelf today provides more certainty in content and form than a century ago.
The Science: Physical & Chemical Properties
Physical properties shape how potassium bisulphate fits into everyday work. Its density sits at about 2.24 g/cm³ and it starts to decompose above 190°C. It dissolves readily in water, releasing a faintly sour tang reminiscent of tart fruit. Chemically, it acts as a reliable acidic salt, donating protons for a range of reactions. Unlike many other sulfates, it resists hygroscopic behavior, so it keeps well in a sealed container without clumping or absorbing much moisture. In the lab, these features spell dependability—no guessing or extra steps to prep a solution, just steady performance when precision counts.
Technical Details and Labeling Practices
Labeling potassium bisulphate has grown stricter over the years. Reputable suppliers list content by percentage, clearly stating moisture levels, heavy metal content, and insoluble matter. There’s steady enforcement of standards—think ACS reagent grade or food-grade checkpoints. Labels must carry hazard info, identifying potassium bisulphate as an irritant with strong acid content. Package sizes range from small bottles for lab use up to drums for industrial buyers, each batch stamped with a lot number and expiry. This rigor increases safety and helps avoid mix-ups in fast-moving production lines.
Preparation—From Theory to Practice
Preparing potassium bisulphate typically starts with reaction between potassium chloride and sulfuric acid. Sulfuric acid, poured into solid potassium chloride, generates potassium bisulphate and hydrogen chloride gas, which must be safely managed since it can corrode lungs and equipment. After the initial batch cools, the result crystallizes into the granular product found on store shelves. Manufacturers test each lot for purity, weeding out excess chloride or unreacted acid. This preparation method traces its roots to laboratory glassblowing, but scaled-up, automated systems now churn out tons daily, feeding industries worldwide right from the refinery floor.
Chemical Reactions and Useful Tweaks
Potassium bisulphate churns out chemical magic once dissolved in water. It releases bisulfate ions that break down proteins, help with organic syntheses, and find a spot in fertilizer blends. Heating it long enough further produces potassium pyrosulfate, which acts as a dehydrating agent or an ingredient in other specialty chemicals. Scientists have even modified its structure to tweak acidity or pair it with other salts, chasing performance for niche catalytic reactions. This flexibility keeps researchers coming back, experimenting with both new blends and tried-and-true recipes in pursuit of better, cleaner processes.
What's in a Name? Synonyms and Branding
Names create confusion for folks stepping outside the chemistry classroom. Potassium bisulphate hides under trade names like arcanite or acid potassium sulfate, and scientific circles sometimes call it potassium hydrogen sulfate. Product names from different suppliers can tack on suffixes for purity or use, such as “food-grade” or “ACS Reagent.” This swirl of terms means reading a data sheet matters, especially when applications shift from brewing to chemical synthesis or water treatment. Consistent terminology helps those of us running experiments—or tracking stocks in a warehouse—stay on the same page.
Safe Handling—More Than Just Good Practice
Handling potassium bisulphate takes more than gloves and goggles. Mistaking it for a neutral salt risks damaged skin, respiratory irritation, or ruined equipment. Safety data sheets recommend acid-resistant gloves, splash proof goggles, and good ventilation. Spills call for immediate neutralization—soda ash or dilute alkali often do the trick. In warehouses, stacking regulations keep drums dry and off the floor, and containers stay sealed against moisture and contamination. Worker training covers safe disposal, since acid sulfates in waste water lead to regulatory fines or environmental headaches. From my own experience, a close call with an unmarked bottle proved labeling and safety checks save more than paperwork—they keep people out of harm’s way.
Where Potassium Bisulphate Gets Used
Wine-makers and brewers reach for potassium bisulphate to stabilize products, preventing wild fermentation and haze. It acts as a cleansing agent, stripping tarnish from metal surfaces before further treatment. Focusing on agriculture, it turns up in fertilizers as a source of potassium and sulfur, balancing plant nutrition without dumping chloride into sensitive soils. Analytical chemists trust its acidity for sample digestions, knowing it gives sharp, reproducible results every run. These wide-ranging uses have become deeply woven into modern production and quality control, cementing the compound’s standing beyond the walls of the science building.
Chasing Better Results: Research and Development
Researchers are pushing potassium bisulphate into new territory. Enzyme chemists test it for protein crystallization, exploring next-gen pharmaceuticals. Green chemistry teams tinker with alternative production—reducing reliance on concentrated sulfuric acid or switching to more sustainable mineral sources. A growing body of work looks at improving solubility and even shifting the particle size for better blending or faster reaction rates. Industry consortia share data on how trace contaminants affect downstream quality, prompting constant refinement. It turns out, even something with decades of steady application gets pulled into today’s race for safer, cleaner, and more efficient solutions.
Digging Into Toxicity—Risks and Realities
Potassium bisulphate carries real risks if handled carelessly. Inhalation of dust or vapors irritates airways and, at high enough doses, can lead to chemical burns or chronic lung issues. Swallowing enough of it brings on gastrointestinal distress, and contact with skin or eyes leads to burns. Animal studies paint a mixed picture—high doses cause tissue damage, but at the levels found in fertilizers or wine, risk is low with proper controls. Regulatory agencies have weighed this evidence, laying down strict exposure limits and cleanup protocols. Anyone regularly working with potassium bisulphate soon learns that a slip in concentration or handling technique can have sharp consequences, reinforcing the value of clear labels and solid protocols over any shortcut.
Looking Ahead—Future Prospects
Researchers and manufacturers keep an eye on potassium bisulphate due to shifting trends in agricultural regulation, green chemistry, and global trade. As countries clamp down on pollutants, non-chloride potassium sources can catch a premium. Increasing demand for minimally-processed foods and natural wine has triggered deeper dives into its role as a preservative, with companies tweaking purity and particle shape to suit new standards. Innovation around alternative synthesis—those that cut use of hazardous reagents or energy-intensive steps—holds promise not just for cost but for cutting the environmental footprint. If experience teaches anything, it’s that a compound like potassium bisulphate, with a long record of safe, effective use, invites experimentation and refinement at every turn, never quite running out of uses as industries keep changing and new challenges keep chemistry relevant.
A Closer Look at a Common Chemical Helper
Potassium bisulphate shows up quietly in daily life and industry. I first ran across it while fixing my home brewing kit, not really expecting to bump into industrial chemistry during that day. Its name sounds technical, but its uses touch everything from winemaking to fertilizers. If you’ve ever sipped wine, enjoyed a flavored juice, or appreciated a crystal-clear bottled drink, potassium bisulphate may have played a part.
Making Wine and Preserving Flavors
Winemakers rely on it to maintain the right acidity. Grapes don’t always ripen with the ideal sugar and acid balance, especially in unpredictable weather, so potassium bisulphate helps set wines up for the aging process. Not balancing those acids would leave wines tasting flat or spoil-prone. The acidification it brings preserves color and stability, cuts down on unwanted bacteria, and keeps off-flavors at bay. In the home kitchen, you’ll find it listed as a food additive, known as E228. It’s common in fruit juices and ciders for the same reasons.
Keeping Food Safe and Appealing
Beyond flavor, food safety comes to mind. Sulfites, which release from potassium bisulphate, bind to oxygen and slow the browning in ready-sliced fruit or dried produce. That fresh-looking apple ring on the store shelf stays bright for longer than nature intended, mostly thanks to this chemical helper. Of course, people with sulfite sensitivity need clear labeling—one study from the FDA in the United States estimates that about one person in every hundred is sensitive, a fact ignored at the food producer’s peril.
Agricultural Boost and Chemistry Workhorse
In my family’s garden, potassium often stands between robust growth and stunted tomatoes. Blended in fertilizers, potassium bisulphate supplies two essential nutrients: potassium and sulfur. These power up strong stems and boost the disease resistance of crops. Modern farming leans hard on these simple chemicals to keep yields up and costs down.
Chemists tank up on potassium bisulphate for other reasons. In labs, it’s a strong acid handy for synthesizing dyes and pharmaceuticals. The acid comes through in manufacture, etching glass or driving reactions that produce other compounds. The chemical industry has depended on it for over a century, and even after all this time, it continues filling a spot on the shelf in almost every teaching or research laboratory.
Challenges and Safer Practices
Use brings responsibility. Mistakes happen when labeling fails or workers skip gloves. It’s irritating if spilled and not great for lungs if powder becomes airborne—this message hits home for anyone who has handled cleaning up a spill without proper gear! Regulatory bodies like the European Food Safety Authority and the U.S. Food and Drug Administration keep tight reins on its allowed concentrations. At home, better transparency—simple ingredient lists and allergy warnings—helps people make smart food choices. Industry leaders invest in better training and monitoring to keep workers safe on the job.
Moving Forward with Better Use
Looking ahead, some companies push for alternatives in shelf-stable foods, focusing on consumer health and sustainability. Better education around food labels and hands-on worker safety training in factories support everyone’s wellbeing. Potassium bisulphate won’t disappear soon, but using it smarter will shape how food and materials get made for the next generation.
What is Potassium Bisulphate?
Potassium bisulphate isn’t the kind of word that pops up in everyday conversation, but it’s more common than it sounds. You’ll find this compound, also called potassium hydrogen sulfate, on food labels as E228. Food manufacturers turn to it as a preservative and an acidifier, giving processed foods a longer shelf life and a slight tang. In smaller doses, it helps wine keep its flavor, and stops dried fruits from turning brown. Science shows it acts by lowering pH, which stops microbes from growing out of control.
How Safe is Potassium Bisulphate?
Most folks aren’t reaching for a spoonful of chemicals. But just because we don’t see the compound in our spice cupboards doesn’t mean we don’t eat it. Potassium bisulphate gets approval as a food additive in many parts of the world, including the United States, Australia, and across the European Union. Food safety agencies, like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), have looked at all the evidence and set safe limits for how much people can eat daily.
Common sense tells us too much of anything can cause problems, and that’s true here. High amounts might upset your stomach or irritate your throat. More seriously, for people who have asthma or a sulphite sensitivity, even tiny bits can cause trouble—think headaches, rashes, or asthma attacks. The World Health Organization set its acceptable daily intake at up to 0.7 mg per kilogram of body weight. In real life, it’d take piles of dried apricots or gallons of wine to go over that limit if you’re an average adult.
Why Does This Matter?
Food safety can feel like something best left to labs and specialists. But I’ve learned that labels are for regular shoppers, parents, cooks, and anyone with allergies. People deserve to know what goes into their food. I grew up with a parent who had a sulphite allergy. Grocery trips meant extra time spent squinting at fine print, weighing the risk of each dried fruit and bottle of juice. People with sensitivities live with these trade-offs every day, so the presence of potassium bisulphate—though harmless for most—really matters for some.
Transparency shapes trust. Food companies have to disclose when they add sulphites to products above a certain level—usually 10 parts per million. That’s a thin line for people with allergies. One bite or sip is all it takes for an adverse reaction. Ensuring that information is visible, clear, and easy to understand is more than a regulatory checkbox. It builds consumer confidence and keeps vulnerable folks safe.
Possible Solutions and Steps Forward
Better labeling makes the most difference. If product labels used bigger fonts, bold warnings, or even simple color coding for major allergens, everyone’s shopping trip would improve, especially for those who need to steer clear of sulphites like potassium bisulphate. Schools, restaurants, and food prep businesses can play a part, too. Educating staff about ingredients, reading supplier documentation, and offering safe alternatives for sensitive diners strengthens the food safety net.
At home, I tend to choose whole foods, not because I’m scared of every additive, but because fresh fruit, vegetables, and home-cooked meals make it easy to avoid things I don’t want to eat. Potassium bisulphate isn’t a cause for concern for most, but it never hurts to read and ask questions. Food should bring people together—not leave anyone out.
Digging Into KHSO4
Potassium bisulphate shows up often in labs and factories, but not everyone stops to think much about what goes into its makeup: KHSO4. That formula packs a punch in classrooms and on the production floor. One potassium atom, one hydrogen atom, one sulfur atom, and four oxygen atoms. It looks simple, yet it pulls a lot of weight in different industries.
Why KHSO4 Matters In Real Life
Back in my chemistry classes, this salt would turn up during titrations, especially when the teacher wanted a strong acid but not sulfuric acid's full force. Potassium bisulphate acts as a handy, solid source of hydrogen ions. That means you get an acidic solution out of a product you can scoop out of a bottle, instead of fussing with concentrated acids.
Out in the world, KHSO4 isn’t just for chemists. Winemakers use it to lower pH without adding sodium, keeping wine’s flavor stable. Glass manufacturers count on it for shaping and finishing. Food companies turn to it for leavening and preservative effects. These uses all depend on its unique chemical structure and how it plays with water and other compounds.
Risks Hidden in Everyday Chemicals
Not every chemical with such a simple formula is free of risk, and this one's no exception. Its acidity helps, but it also carries a sting for skin or eyes. Breathing in the dust or getting KHSO4 in a cut can spell out a bad afternoon quick. Businesses using potassium bisulphate have clear guidelines to train workers and label containers—OSHA rules help here. I’ve seen folks skip gloves only to regret it later, no matter how common a compound might seem.
This brings up one point often forgotten—usefulness doesn't cancel out the need for respect. Chemicals deserve care and understanding for both their value and their hazards. Potassium bisulphate won’t explode or catch fire, but it can hurt unprotected hands and eyes. That’s why training counts just as much as a chemical’s formula.
Finding Safer and Smarter Solutions
Some places experiment with swapping out potassium bisulphate for milder salts or adjusting recipes to lower the total amount used. This comes up a lot in food production, especially where consumers ask for simpler ingredient lists. Careful research looks into substitutes that keep the job done without upsetting product safety or quality. The goal is pretty straightforward: use the right amount, train the workers, and consider safer alternatives when performance doesn’t drop off.
Education matters, too. High school and college students need hands-on guidance, not just numbers and letters scribbled on the board. Real-world practice with safety gear, handling spills, and understanding chemical reactions builds respect for even basic compounds like KHSO4. Sharing stories about careless moments and small accidents sticks in the mind much longer than reading rules alone.
Final Words on a Common Compound
So, the formula KHSO4 belongs to more than textbooks. It sits on shelves, in factories, and inside food packages. Its story runs through chemistry labs, industrial sites, and even into your kitchen, each place relying on solid facts and a steady hand. Staying curious about such a simple arrangement of atoms makes for smarter choices and safer results—all from a compound with just four types of atoms on the label.
Why Storage Matters for Potassium Bisulphate
Anyone who has worked in a lab knows chemical storage often gets overlooked until something goes wrong. Potassium bisulphate, with its white, crystalline look, might seem pretty unremarkable, but improper storage can set off all kinds of problems. Let’s face it, no one wants an unnecessary lab mishap or ruined batch because a simple storage rule got skipped.
This compound reacts with water and can corrode packaging or shelving over time. On top of that, it releases sulphur oxides in contact with humid air—both annoying and hazardous. I’ll say from direct experience: cut corners here, and you pay for it in stink, corrosion, and risk during cleanup. Just because it isn’t explosive doesn’t mean it deserves any less attention.
Key Storage Considerations
Keep potassium bisulphate dry. Even short-term dampness can lead to clumping or breakdown of the substance. I once watched five kilos turn into a solid brick because someone forgot the desiccant. Use a tightly sealed container—polyethylene or glass both fit the bill. Old plastic bags split open, so don’t use them.
Store it in a dedicated chemical cabinet, and avoid placing it near acids, bases, or anything with reducing properties. Although potassium bisulphate doesn’t go off like fireworks, a reaction with the wrong neighbor still brings headaches—think unexpected vapors, discoloration, or shelf corrosion.
Keep it away from direct sunlight and high temperatures, not because potassium bisulphate becomes more dangerous, but because containers degrade faster under those conditions. Dim, cool, and dry describe nearly every chemical storeroom for a reason, and it fits here as well.
Labeling and Inventory: Don’t Guess
Every jar or container deserves a clear, dated label. Include the name (in full), hazard symbols, and date received. From firsthand experience, it’s easy to mix up lookalike powders if someone slaps on a half-peeled sticker with faded writing, especially at year-end when new stock arrives quickly.
Regular inventory checks catch problems fast. Make a habit of opening the cabinet every few weeks. Condensation inside a jar or powder stuck to the lid means moisture got in. Dispose of compromised material through appropriate waste services, and never just dump it.
Ventilation and Spill Clean-up
Storage should happen in a well-ventilated area, but you don’t want open air swirling into your containers. Ventilation helps if a spill occurs or a container leaks. I’ve had to clean up spills in spaces without good airflow, and irritation in the eyes or nose follows close behind. Use a chemical-rated mask and gloves, sweep up using a disposable scoop, and gather the waste in a sealed bag for safe disposal.
Toward Better Practices
There’s also a lesson in preparedness. Every new lab member deserves training in chemical storage—not just a quick walk-through, but real focus on what each compound needs. A strong system for chemical hygiene helps avoid emergencies. Don’t just leave storage to the most junior staffer. Assign someone with experience to oversee chemical inventory and storage practices.
Manufacturers like Sigma-Aldrich and Fisher Scientific spell out storage guidelines in their datasheets, and every workplace would do well to print and post those instructions. Real safety grows from strong habits, not shortcuts—and a dry, labeled, sealed bottle is the best start for potassium bisulphate.
Why Do We Use Potassium Bisulphate?
Step into most pantries or wine cellars and you’ll find preservatives that help keep food and beverages fresh. Potassium bisulphate shows up in wines, dried fruits, and even some baked goods. Think of it as an insurance policy against spoilage, as it stops microbes from turning grapes into vinegar or mold from taking over a batch of raisins.
Allergic Reactions: Real Problem or Rare Fluke?
Walking through a grocery aisle, the words “contains sulphites” often appear on labels. Sulphites include chemicals like potassium bisulphate. For most people, these compounds don’t cause trouble. But those with sulphite sensitivity can experience anything from hives to tough, wheezy breathing. The FDA estimates one out of every hundred people reacts badly, but individuals with asthma face a higher risk. That means folks with underlying lung concerns shouldn’t shrug off the warning.
The science backs this up. Peer-reviewed medical literature describes everything from mild skin rashes to serious anaphylaxis after sulphite exposure. Stories from allergy clinics pile up too—patients develop headaches, stomach issues, or even life-threatening swelling after eating or drinking products containing this additive. That personal side of the story matters. If you’ve watched a friend scramble for an inhaler at a dinner party, the risk feels much closer to home.
Hidden Sources and Personal Experience
What can catch people off guard is how many products carry these preservatives. I learned from a friend who spent years dodging headaches after wine before realizing sulphites were to blame. After a process of trial and error, she started scanning labels and opting for low-sulphite varieties. Her story isn't rare. Even people without a diagnosed allergy sometimes notice mysterious headaches, rashes, or bellyaches after certain foods. In many cases, potassium bisulphate flies under the radar, making symptoms all the more confusing.
Protecting Yourself: Knowledge and Action
Connect the dots between your body’s signals and what’s in your food. If asthma runs in your family, take a closer look at ingredients. Reading food labels remains a simple way to protect yourself. The FDA requires companies to flag sulphites if they’re present above ten parts per million. That’s not foolproof—imported foods and restaurant dishes slip through the cracks. Restaurants aren’t always upfront if they dust salads or cut fruit with sulphites to keep them looking fresh.
Doctors play a critical role in untangling these symptoms. If you suspect a link between certain foods and strange reactions, a board-certified allergist can help. Skin or blood tests, along with a careful food diary, often reveal the culprit. Bringing accurate, science-based information to the table allows people to sort out fact from fear. Chemists and medical researchers continue studying how and why these sensitivities arise, recognizing the need for improved screening and clearer labelling.
What Can Change?
Better labeling and more public awareness solve half the problem. Pushing for clearer warnings on restaurant menus or imported products helps those with allergies eat out safely. Food makers should experiment with alternatives—natural preservation methods lower the risk for everyone, not just those affected. Empowering shoppers with real data produces healthier, safer meals for families who deserve more transparency than ever before.
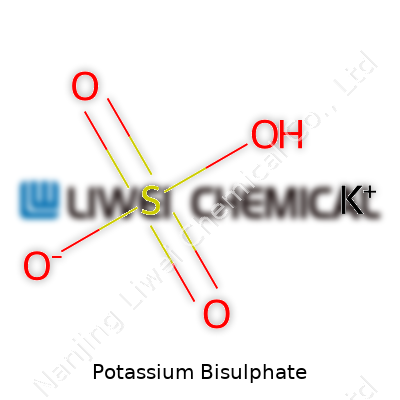
| Names | |
| Preferred IUPAC name | Potassium hydrogen sulfate |
| Other names |
Potassium hydrogen sulfate
Potassium hydrogen sulphate Potassium acid sulfate Potassium acid sulphate Monopotassium sulfate Monopotassium sulphate |
| Pronunciation | /poʊˌtæsiəm bɪˈsʌlfeɪt/ |
| Identifiers | |
| CAS Number | 7646-93-7 |
| Beilstein Reference | Beilstein Reference 3587158 |
| ChEBI | CHEBI:1319 |
| ChEMBL | CHEMBL1201591 |
| ChemSpider | 21516 |
| DrugBank | DB14045 |
| ECHA InfoCard | 100.029.682 |
| EC Number | 231-594-1 |
| Gmelin Reference | Gmelin Reference: **1743** |
| KEGG | C06744 |
| MeSH | D011098 |
| PubChem CID | 24462 |
| RTECS number | TT2975000 |
| UNII | 3KX378VT3C |
| UN number | UN2464 |
| Properties | |
| Chemical formula | KHSO4 |
| Molar mass | 136.17 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.24 g/cm³ |
| Solubility in water | 110 g/100 mL (25 °C) |
| log P | -4.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.99 |
| Basicity (pKb) | 11.6 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.435 |
| Dipole moment | 1.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 202 J K⁻¹ mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1177 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1320 kJ/mol |
| Pharmacology | |
| ATC code | A12BA02 |
| Hazards | |
| Main hazards | Oxidizing, harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | Hazard statements: "H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-2-Acide |
| Explosive limits | Explosive limits: Non explosive |
| Lethal dose or concentration | LD50 (oral, rat): 2340 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Potassium Bisulphate: 2,340 mg/kg (rat, oral) |
| NIOSH | WN3875000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Potassium Bisulphate: "Not established |
| REL (Recommended) | 2.6 |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Potassium sulfate
Potassium persulfate Potassium metabisulfite Potassium hydrogen sulfate Sodium bisulfate |

