Hexachlorocyclotriphosphazene: A Closer Look
Historical Development
The story of Hexachlorocyclotriphosphazene began in the late 19th century, woven into the early days of polymer chemistry. Chemists uncovered this molecule in the laboratory, pushing beyond conventional organic compounds to explore phosphorus-nitrogen rings. At first, applications remained academic. In the mid-20th century, industry’s attention to flame retardants and specialty plastics made Hexachlorocyclotriphosphazene’s unique backbone truly valuable. Since then, its use has grown in step with the expanding world of advanced materials. Researchers built upon older, small-batch syntheses to scale up production during the postwar boom, responding directly to real-world needs in electronics, aerospace, and specialty polymers.
Product Overview
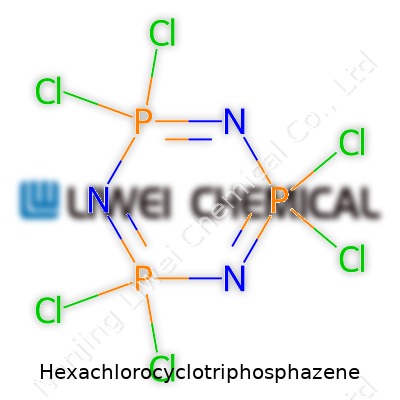
Hexachlorocyclotriphosphazene stands out as a six-membered ring consisting of alternating phosphorus and nitrogen atoms, each phosphorus atom carrying two chlorine atoms. Its formula—N3P3Cl6—gives it a distinctive fingerprint chemists can identify quickly on a spectrum. Commercial products come in crystalline or powder forms, often with purity upwards of 99%. Users in manufacturing, R&D, and academia watch for batch consistency, as trace impurities can throw off results in sensitive reactions.
Physical & Chemical Properties
This compound forms a white or slightly yellow powder, melting around 114 °C and boiling near 156 °C, where it distills without decomposition under reduced pressure. Hexachlorocyclotriphosphazene dissolves well in aromatic solvents and chlorinated hydrocarbons but resists water; hydrolysis occurs, especially with heat or in the presence of alkaline catalysts, slowly breaking it down. Its P–N ring holds up under moderate heat, making it valuable in thermally stable plastics and resins. In the air, it keeps well in sealed containers, but moisture prompts gradual breakdown, so handling in dry, inert atmospheres safeguards its integrity during storage and use.
Technical Specifications & Labeling
On technical data sheets, you’ll find the CAS number 940-71-6 printed alongside identification codes. Producers specify melting and boiling points, minimum purity (usually above 99%), and moisture content below 0.5%. Container labeling highlights hazards like respiratory and skin irritation, referencing GHS classification. Safe storage guidance calls for cool, dry areas, tightly sealed packaging, and clear date stamps to track shelf life. These specs give purchasing agents and lab managers confidence that what arrives matches expectations, with proper traceability if a problem comes up later.
Preparation Method
Large-scale synthesis depends on reacting phosphorus pentachloride (PCl5) with ammonium chloride (NH4Cl) under controlled heat. Early chemists ran the process in sealed tubes, but today’s factories use improved reactors for better yields and reproducibility. The key step—cyclization—generates the six-membered ring and drives off side products, so reactor design ensures complete mixing and temperature control. Automation and solvent selection play a big role in safety, as runaway reactions make industrial production risky. Recovered product gets purified through recrystallization or vacuum distillation, removing unreacted feedstock and chlorinated byproducts.
Chemical Reactions & Modifications
This molecule’s greatest strength comes from those six chlorine atoms, which swap out easily with all sorts of nucleophiles—alcohols, amines, or phenols—giving chemists a huge toolbox for making custom polymers. For instance, reacting Hexachlorocyclotriphosphazene with ethylene glycol produces elastomers, while treatment with aromatic amines creates flame-retardant fibers. Scientists have tinkered with these reactions for decades, finding new ways to tune final properties: improved flexibility, tougher backbones, or tailored electrical characteristics. This versatility keeps Hexachlorocyclotriphosphazene relevant wherever performance materials are needed.
Synonyms & Product Names
In the literature and on supply lists, you may also spot names like trimeric phosphonitrilic chloride, phosphonitrilic chloride trimer, or NPC trimer. Some catalogues use the shorthand HCCP or simply cyclotriphosphazene. Certain chemical distributors assign trade names such as Phosphonex 600 or PNCT-3, meant to highlight specialty applications like coatings or adhesives. These alternative labels can cause confusion with newcomers, especially in international settings, but the unique structure links all these terms back to the same core compound.
Safety & Operational Standards
Anyone handling Hexachlorocyclotriphosphazene wears safety goggles, gloves, and lab coats, and works with local exhaust or ventilated fume hoods. Its dust can irritate eyes, skin, and the respiratory tract, and contact with moisture or acids produces hydrogen chloride—a corrosive, pungent gas. Emergency procedures spell out what to do in the event of a spill or exposure: flush skin with water, evacuate affected zones, and avoid inhaling vapors. Shipping regulations classify the product as hazardous, so drums, bottles, and bulk containers bear proper UN codes and handling instructions. Training plays a crucial part in keeping accidents rare and ensuring that workers respect the material’s potential dangers.
Application Area
Industries turn to Hexachlorocyclotriphosphazene for its role in specialty plastics, especially where thermal or flame stability matters. In wire and cable sheathing, its derivatives prevent fire propagation. Aerospace and automotive parts use polyphosphazenes for insulation and gaskets that must survive high heat and mechanical stress. Medical device makers value the tunable biocompatibility of modified phosphazene elastomers for catheters and implants. Research outfits develop novel membranes based on this backbone for fuel cells and separation technologies. Even electronics firms include its compounds in printed circuit boards, mixing durability with lightweight build. This broad utility means demand keeps growing, especially wherever safety regulations favor fire-safe, robust components.
Research & Development
Chemists and engineers never seem to stop exploring new modifications of the phosphazene ring. Polymer science journals bristle with recipes for hybrid materials: incorporating nanoparticles, biological molecules, or novel side chains. Today’s R&D aims for membranes with ultra-selective gas separation, advanced flame retardancy with minimal smoke, and coatings for corrosion-prone metals. Strategic partnerships—linking universities, companies, and government labs—have yielded stronger, more processable versions of the parent molecule, and have found ways to recycle or safely degrade old phosphazene polymers. Continued innovation pivots on a mix of synthetic ingenuity and better analytical tools, as research trends respond to shifting industrial and environmental priorities.
Toxicity Research
Comprehensive toxicological studies remain limited, though acute dust exposure often brings respiratory distress. Animal testing revealed possible mild chronic effects with repeated high-level dosing; rodents exposed to phosphazene derivatives sometimes showed liver or kidney strain, but these findings seldom translate cleanly to industrial environments, where exposure is lower and controls stricter. Researchers continue to monitor for subtle environmental persistence, since halogenated compounds sometimes bioaccumulate. Most current assessments mark Hexachlorocyclotriphosphazene as hazardous but manageable if facilities uphold ventilation requirements, personal protective gear, and regular staff training. Environmental groups push for even tighter exposure limits, especially near production plants, aiming to prevent spillover into water or soil.
Future Prospects
As resource efficiency and fire safety move up the regulatory agenda, Hexachlorocyclotriphosphazene’s flexible chemistry points to a rising demand. The growth of electric vehicles, green buildings, and renewable energy systems all seek better materials for wire insulation, flame barriers, and ion-conducting membranes. Research into biodegradable or easily recycled phosphazenes challenges producers to balance performance with eco-friendly lifecycles. Machine learning and molecular design software accelerate the search for new side chains or functional groups. More streamlined manufacturing—lower waste, less toxic byproducts—will draw investment from bigger players in Asia and North America. Real opportunity lies in blending lab innovation with practical engineering, ensuring Hexachlorocyclotriphosphazene stays central to the next generation of performance polymers.
Not Just Another Lab Chemical
Hexachlorocyclotriphosphazene doesn’t exactly roll off the tongue, but it plays a quiet role in a lot of things we use every day. I remember hearing the word from a friend who works in insulation manufacturing. At first, it sounded like an obscure compound from a textbook. It turns out, this little molecule, usually called HCCP for short, has found its way into more real-world applications than most folks would guess.
Shaping the Plastics and Electronics World
Walk through any electronics shop and odds are, you’ll see gadgets protected with fire-resistant casings. HCCP often sits behind the scenes in these applications. By mixing into plastics, it helps products meet strict fire safety rules. Old stories from factory lines show that without something to slow down flames, plastics melt and electronics quickly fail in the event of a short circuit or surge. A few years ago, tougher legislation meant manufacturers had to find non-halogenated flame retardants — substances that limit the spread of fire, but don’t produce as many toxic byproducts. HCCP’s unique phosphorus-nitrogen structure steps up to the task, cutting the risk of catastrophic fires.
Plastics made with HCCP not only resist heat, but also block out moisture. This means insulation stays effective in electrical equipment on rainy days or in stuffy server rooms. As someone with a background in tech repairs, I’ve seen old circuit boards with water-damaged traces and warped sleeves. Stronger, more water-resistant plastics mean longer-lasting electronics and safer operations in hospitals, vehicles, and even satellites.
Helping Make Specialty Rubbers and Fibers
Tires, conveyor belts, hoses — all rubber-based products need to survive a harsh mix of stress, weather, and chemical exposure. HCCP shows up as a crosslinking agent, basically acting like the knots in a fishing net that hold the whole thing together. That extra toughness gives rubbers their bounce and durability. In textiles, especially high-performance fibers used for things like firefighter gear or space suits, HCCP brings in flame resistance, so synthetic fabrics don’t catch fire as easily in extreme conditions.
Role in Pharmaceuticals and Water Treatment
Sometimes, chemists use HCCP to build more complex molecules. The phosphazene ring lets them attach different chemical groups, kind of like decorating a Christmas tree with your favorite ornaments. In medicine, this has led to new drug development paths, especially for antiviral or anticancer agents. Companies working on advanced water filters also take advantage of HCCP since it bonds to filter membranes, capturing contaminants that other materials let slip by. This keeps taps cleaner and water quality higher, especially in places where industrial runoff is a concern.
Why This Matters Today
Growing regulations around hazardous chemicals keep putting pressure on developers to look for safer, more reliable solutions. HCCP’s relatively low toxicity and adaptability steer industries in a more sustainable direction. But risks remain — mishandling or improper disposal can pollute soil and water. It’s not just about switching from one chemical fix to another. Teams designing products with HCCP need good training, updated safety protocols, and pressure from watchdog groups to make sure they treat this chemical with respect from factory to landfill.
After seeing the difference safer materials make, it’s easy to appreciate both the science and responsibility behind using compounds like HCCP. Cleaner fires, tougher materials, and purer water all start with choices made inside a chemist’s lab.
Chemical Formula: N3P3Cl6
You don’t hear about hexachlorocyclotriphosphazene much at neighborhood gatherings, but students, chemists, and product engineers know this molecule by heart: N3P3Cl6. With twelve atoms rattling around a six-membered ring structure—three nitrogen, three phosphorus, topped with six chlorine—it’s a chemical puzzle piece that snaps into a number of scientific projects.
Why This Compound Stands Out
I first came across this formula during a stint at a materials science lab, working on fire-resistant coatings. The unique phosphorus-nitrogen backbone offered a springboard for resin development. Hexachlorocyclotriphosphazene’s repeatable ring core gives it stability and a capacity for chemical modification, both of which have earned it a spot in polymer chemistry toolkits.
Researchers like it because swapping out the chlorine atoms with other groups opens up a world of possible derivatives. This basic skeleton forms the basis of a whole family of phosphazene compounds. The properties shift depending on what’s attached. That adaptability matters in electronics, coatings, adhesives, and even biomedical fields. For example, some of the resulting polymers shrug off flame and resist chemical attack, making them useful for extreme environments—think aerospace and advanced electronics.
Safety, Sustainability, and Doubt
As just about anyone involved with major chemicals knows, practical uses don’t come without headaches. The production, storage, and ultimate disposal raise real questions. Chlorine doesn’t carry the friendliest reputation, and accidental release can endanger people and the ecosystem. Strict protocols surround its use, adding cost and complication. Some researchers push for alternatives or call for careful lifecycle analyses before mass adoption.
I’ve seen more labs prioritize “greener” chemistry in the past decade. Substituting less hazardous reagents or recycling waste streams gets attention. Regulations nudge companies to tighten up practices, both in the manufacturing space and downstream product handling. These aren’t quick fixes, but steady improvement marks responsible progress.
Supporting Facts
Hexachlorocyclotriphosphazene's popularity in research circles stems from its versatility. Around 15,000 tons are produced globally each year, and the demand ties directly to the rising need for specialty polymers. According to academic studies published in Advanced Materials and Polymer Chemistry, substituting out the chlorines with organic side groups converts the molecule into polymer building blocks. In real-world terms, these are used in circuit board laminates, fire retardants, and medical devices.
Despite all its promise, ease-of-production is no walk in the park. High-purity manufacturing needs tight quality control. Supply problems and environmental rules sometimes drive prices up, motivating some to look at bio-based alternatives for select applications. Researchers in Germany and Japan recently demonstrated that renewable phosphorus sources could enter this supply chain—the science isn’t there yet at commercial scale, but momentum is building.
Possible Solutions
Keeping chemistry sustainable demands taking the long view. Investing in better containment, safer substitutes for chlorine, and stronger recycling infrastructure helps tamp down risks. In my old lab, closed systems and scrubbers dealt with escapes, keeping both users and neighborhoods safer. Most progress happens when chemists, engineers, regulators, and end-users all compare notes and adapt as needed. No single group carries all the answers, but each step hikes up collective experience.
Hexachlorocyclotriphosphazene, with its N3P3Cl6 structure, keeps proving its worth while bringing challenges of its own. The ongoing work to handle it wisely says a lot about the innovation and responsibility needed in modern materials science.
Understanding What We’re Dealing With
Hexachlorocyclotriphosphazene, often recognized by chemists for its tough-to-pronounce name, shows up in fire retardants, specialty plastics, and even the coatings used in electronics. Over the years, this ring-shaped compound has carved out a niche in industry because its structure can handle tough jobs in aggressive environments. But real questions surround what happens to workers, end-users, and nearby communities every time this chemical enters a factory or leaves it as waste.
Health Concerns for Workers and the Environment
I’ve watched people working in chemical plants and labs, gloves sticky and masks snug, moving containers marked with hazard symbols that aren’t just there for show. Direct exposure to Hexachlorocyclotriphosphazene can irritate skin, eyes, and airways; internal exposure through swallowing or longer-term breathing can threaten organs. The Environmental Protection Agency lists chlorinated phosphazenes as toxic, especially in workplaces lacking strict controls. Chlorine atoms in this molecule tend to make reactions unpredictable, sometimes producing dangerous byproducts like hydrochloric acid gas when things go wrong. That threat hangs over not just the folks handling the pure chemical, but every person nearby if ventilation fails or leaks spring up.
Community health can take a hit, too. Water near chemical manufacturing plants sometimes picks up traces of phosphazene waste. In large doses, aquatic life can be wiped out—which is no small story in places where rivers provide drinking water and food. Regulators track these releases, but enforcement gaps leave holes that real people have to fill with phone calls, activism, and, at times, doctor visits for symptoms that don’t go away. People living near these plants carry the burden, not just lab technicians in masks and goggles.
Weighing Risk Against Industrial Benefits
Plastics and coatings laced with Hexachlorocyclotriphosphazene offer unique fire resistance and durability. It’s not just about comfort—these products can save lives in buildings and electronics by slowing the spread of flames. Then again, tradeoffs add up. Once in a product, the chemical sits locked away, for the most part, but accidents and disposal can break that lock wide open. The “hazardous or toxic” label sticks because of the trouble it causes when things run off track. Plenty of safer alternatives exist in theory, but cost and inertia keep the industry tied to what already works, even if it leaves risky leftovers behind.
Smarter Choices in Handling and Substitution
I’ve spoken with engineers who believe switching to modern fire retardants, which break down faster and pose less danger to humans and wildlife, can help. Industry can cut risks by updating equipment for leak detection and running finer filters at discharge points. Clear labeling and transparent safety data sheets support busy workers who shouldn’t gamble with their health for a paycheck. Local authorities and the companies making this stuff both play a role, and open conversations with impacted communities lead to pressure for better safeguards. Mistakes in the past, where hazardous substances slipped into drinking water or soil, teach us that hiding behind technical language only ends in community mistrust and higher cleanup costs down the line.
Stepping Forward With Caution and Care
No one expects to ban every useful chemical, but seeing the real risks behind Hexachlorocyclotriphosphazene pushes for upgrades in both rules and everyday work habits. Personal experience dealing with chemical accidents in labs sticks with you: even skilled professionals can’t always dodge the dangers if shortcuts get normalized. Smart regulation, protective gear, and fresh investment in safer technology offer a better way forward. Businesses, workers, and neighbors all have skin in the game. Paying close attention to chemicals like this one helps everyone move toward cleaner air, safer water, and better health.
Recognizing Real Risks
Hexachlorocyclotriphosphazene doesn’t look all that threatening in a jar. Still, this chemical comes with a track record—stinging eyes, irritated skin, and strong fumes. If it lands on the ground in a humid spot or spills in the lab, things turn messy fast. Moisture leads to decomposition, and toxic hydrochloric acid emerges in the process.
Many labs keep phosphazene compounds for their use in flame-retardants and specialty plastics. The thing is, storing this stuff in a careless manner can invite long-term headaches. Corrosion damages shelves and containers, off-gassing makes life miserable, and in worse cases, people get hurt. My work with specialty chemicals showed me how easy it is for safety steps to slip once everyone feels familiar with the job. All it takes is one overlooked container or wet glove for avoidable accidents to show up.
Choosing the Right Storage
A dry spot, far from moisture, supports safety here. Polyethylene or Teflon bottles handle the chemical without breaking down, so glass or metal jars won’t last. Screw caps with good seals stop vapors and keep humidity out—a must in damp climates or steamy work areas. I remember opening a loose cap after a summer weekend, getting a sharp whiff, and realizing firsthand why tight lids make a difference.
Labeling stands out in my memory. Too many people grab from "clear jars" assuming all white powders act the same. Big, bold writing and a warning sign backed by a dated log of checks, show everyone what they’re dealing with. Mix-ups lead to poor handling or surprise reactions in waste containers—real consequences for rushed labs.
Good Practice Saves Skin
Gloves and goggles seem obvious, but a steady pace with every transfer works even better. Nitrile gloves block contact, while long sleeves and a chemical apron keep splashes off the skin. I’ve watched new staff handle chemicals like this one while half-focused—either chatting, rushing, or juggling other tasks. Spills happen during those lapses. Slow, quiet work reduces both spills and injuries.
Engineering controls make a real-life impact. A well-maintained fume hood stops vapors from drifting. Simple habits like working under the sash, never reaching above reaction vessels, and checking airflow go further than printed rules that no one reads. These steps absorbed into the routine, rather than an afterthought, bring results.
Planning for the Inevitable Mistake
People drop bottles. Pumps fail and splashbacks catch you unaware. Quick access to an eyewash station and emergency shower limits harm. A chemical spill kit near the workbench, stocked with inert absorbents and neutralizing agents, helps clean up before trouble spreads. On days when everything goes smoothly, these supplies don’t get touched. But once or twice a year, one gets grateful they were stocked within reach.
The Benefits Are Worth the Trouble
No chemical safety guideline feels urgent until an incident ruins a workday. Storing and handling hexachlorocyclotriphosphazene with respect keeps people out of the emergency room, maintains lab integrity, and protects costly equipment. Rushing, guessing, or trusting labels from last year opens the door to risks. Clear routines, solid containers, and attention in the moment do more to keep folks safe than any policy binder on the shelf. That’s my experience on the front line—and I value peace of mind over shortcut time savings, every time.
Where Hexachlorocyclotriphosphazene Finds a Home
Hexachlorocyclotriphosphazene might sound like a tough chemical to pronounce, but it gets plenty of hands-on use in the real world. The first time I came across this compound was during a visit to a specialty plastics plant, and I realized it plays a much bigger role than most folks notice. Polymeric chemistry drives big changes in fields like electronics, coatings, and even fire protection. In each of these sectors, practical results matter more than fancy terminology.
Electronics Manufacturing Relies on its Durability
If you dig around the back of a computer or pop open a circuit board, you’ll see components protected by laminate materials. That tough, shiny layer is there for a reason: it blocks heat and stops electricity from wandering where it shouldn’t. Hexachlorocyclotriphosphazene forms the base in many of these protective layers thanks to its strong resistance to both flames and high voltage. Printed circuit boards in consumer electronics often use it as a base to keep devices safer for longer. Flame-retardant capability isn’t a small matter in a world full of chargers and batteries that heat up under pressure.
Coatings and Advanced Materials
Take the coatings sprayed inside aircraft cabins or on car components—many include derivatives of this phosphazene because it stops plastic from catching fire and keeps out unwanted moisture. I’ve talked to engineers who tested coatings both with and without these additives. The difference shows up quickly in lab burns and water submersion tests. This chemical helps companies meet safety standards without weighing down parts, and that translates to lighter, more efficient cars and planes.
Fire Retardants for Textiles and Construction
Fire marshall reports often point out how rapidly flames travel on unprotected fabrics. Hexachlorocyclotriphosphazene enters the textile chain by giving drapes, carpet, and upholstery a flame-slowing boost. You’ll also find it in foams and construction materials—plasterboard and insulation panels become more reliable barriers, buying people valuable extra time in case of fire. Seeing house fires slowed by modern materials brings home how much this chemical helps in daily life.
Specialty Rubber and Elastomers
Rubber seals and gaskets keep machinery ticking by blocking leaks and absorbing vibration. Phosphazene-based rubbers handle chemical spills, high temperatures, and fatigue much better than natural rubber or old-style synthetics. Factories making seals for oil rigs and chemical plants depend on this reliability. I’ve visited supply rooms where maintenance crews swear by these materials, knowing that leaks bring costly shutdowns.
Sustainability and Safety Considerations
Any industrial chemical draws scrutiny for its environmental impact. Responsible manufacturers put strong waste-handling rules in place, making sure hexachlorocyclotriphosphazene doesn’t enter waterways or soil. Some companies have started researching ways to reclaim and recycle process wastes. Developing safer, closed-loop production remains a challenge worth tackling. As chemistry pushes forward, balancing practical results with safety demands honest work from scientists, managers, and frontline workers alike.
Moving Forward
This compound continues to push what’s possible in electronics, automotive, aerospace, and building safety. Protecting people and equipment keeps teams motivated to improve both performance and sustainability. Technical progress often comes in small increments—each material gives us a chance to build a safer, more comfortable world, if we remember to keep risks in check.
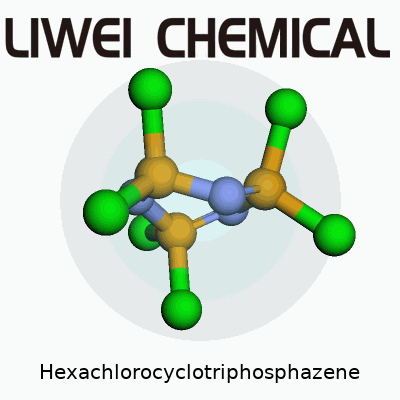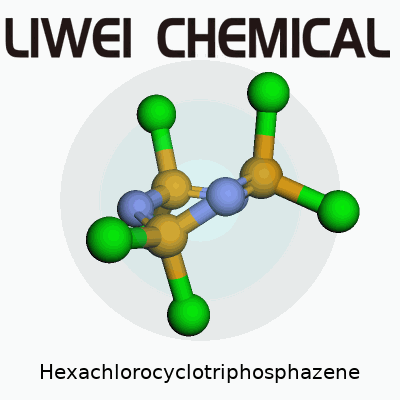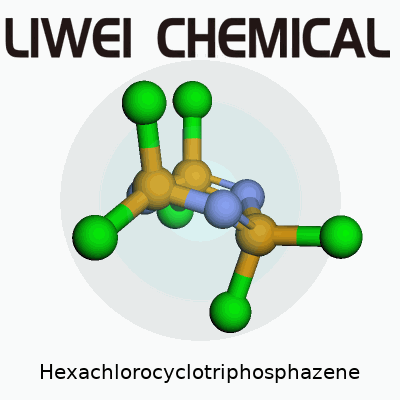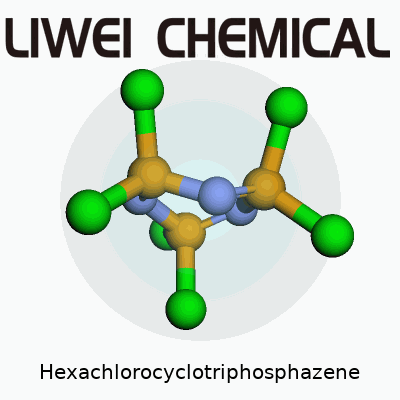
| Names | |
| Preferred IUPAC name | 2,2,4,4,6,6-Hexachloro-1,3,5,2,4,6-triazatriphosphinine |
| Other names |
N-Trichlorophosphazene
Phosphonitrilic chloride trimer Hexachlorocyclotriphosphazane |
| Pronunciation | /ˌhɛk.səˌklɔː.roʊˌsaɪ.kləˌtraɪˈfɒs.fəˌziːn/ |
| Identifiers | |
| CAS Number | 3317-36-8 |
| 3D model (JSmol) | ``` P3N3Cl6 ``` |
| Beilstein Reference | 131107 |
| ChEBI | CHEBI:39070 |
| ChEMBL | CHEMBL1230461 |
| ChemSpider | 21251 |
| DrugBank | DB11440 |
| ECHA InfoCard | 01d31f6e-bd5c-492d-995a-daa42c272c52 |
| EC Number | 206-540-6 |
| Gmelin Reference | 7463 |
| KEGG | C11268 |
| MeSH | D006676 |
| PubChem CID | 8303 |
| RTECS number | MN0875000 |
| UNII | ZG3R5X0VWE |
| UN number | UN2811 |
| Properties | |
| Chemical formula | N3P3Cl6 |
| Molar mass | 403.24 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.97 g/cm³ |
| Solubility in water | 0.00183 g/100 mL (18 °C) |
| log P | 5.16 |
| Vapor pressure | 0.00019 mmHg (25 °C) |
| Acidity (pKa) | 15.0 |
| Basicity (pKb) | 11.32 |
| Magnetic susceptibility (χ) | -88.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.635 |
| Viscosity | 1.4 mPa·s (at 55 °C) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 315.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1593 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3804 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H319, H410 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P314, P321, P362+P364, P337+P313, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3 1 0 |
| Autoignition temperature | 515°C |
| Lethal dose or concentration | LD50 oral, rat: 640 mg/kg |
| LD50 (median dose) | 640 mg/kg (rat, oral) |
| NIOSH | NA8300000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Hexachlorocyclotriphosphazene is not specifically established by OSHA or major regulatory agencies. |
| REL (Recommended) | 0.5 mg/m³ |
| Related compounds | |
| Related compounds |
Hexachlorocyclotriphosphazene trimer
Octachlorocyclotetraphosphazene Phosphonitrilic chloride Polydichlorophosphazene Tetrachlorocyclodiphosphazene |

