Copper Chloride Dihydrate: Looking at an Unsung Chemical Worker
Historical Development
Copper compounds stretch their roots deep into the story of human progress. Alchemists fiddled with copper salts centuries ago, always trying to tease out new colors and strange crystals from base minerals. Early records trace copper chloride’s discovery to the experiments of Andreas Libavius in the late sixteenth century. More systematic research in the nineteenth century mapped out its hydrate forms; copper chloride dihydrate, pale blue-green and easy to work with, emerged from the crystallization habits that chemists observed during evaporations and solution work. Before high-tech labs, craftsmen saw copper chloride show up in dye vats and even magic tricks—its color change under flame was as delightful then as it is today in “flame test” classroom demos.
Product Overview
In its simple form, copper chloride dihydrate comes as glimmering blue-green crystals, easy to scoop from a jar, yet packed with uses across several industries. It’s made by neutralizing copper(II) oxide or copper(II) carbonate with hydrochloric acid, a straightforward approach that creates a steady supply for labs and factories. The formula stays consistent: CuCl2·2H2O. Storerooms rarely run out of this basic ingredient, as it sits near other copper salts for quick grabs. Whether for small-batch synthesis or large-scale metal etching baths, this chemical shows up as a reliable building block.
Physical & Chemical Properties
Copper chloride dihydrate’s blue-green color makes identification simple, but look past the surface and more details emerge. The crystals dissolve easily in water, and the solution releases a faintly sharp, chlorinated scent. Heat it, and the crystals lose water, shifting to a brownish shade as the anhydrous form takes over. Solubility comes in at about 69 grams per 100 mL of water at room temp—no big struggle getting it into liquid phase. The compound stays stable in dry air but picks up water again if humidity rises. It handles gentle acids and bases well, continuing to show its resilience in a variety of settings.
Technical Specifications & Labeling
Suppliers slap labels on copper chloride dihydrate according to purity, water content, and physical form. Typical grades offer minimum purity of 99% for analytical work, though technical grades exist for less demanding tasks. Packaging ranges from powder in plastic bottles to moisture-proof tubs of crystalline chunks. Each label includes hazard symbols, instructions for safe handling, batch numbers, and details about the chemical’s shelf life. In the lab, quality control checks focus on iron, sodium, and other trace metals that could throw off experiments or industrial runs. Reliable suppliers submit certificates of analysis alongside shipments.
Preparation Method
Production happens most often by reacting copper(II) oxide or copper(II) carbonate with hydrochloric acid, driving off carbon dioxide (if carbonate is used) or simply fizzing out in clear solution (with oxide). Chemists then filter away undissolved impurities, heat slightly to dissolve everything, then slowly let the liquid cool. Crystals form as the solution sits, and careful drying at lower temperatures ensures the right “dihydrate” structure forms and sticks around. This classic route delivers on both cost and consistency—making it the top method in both classroom demonstrations and economic scale-up runs.
Chemical Reactions & Modifications
Mixing copper chloride dihydrate with different reagents turns the solution into a playground for color changes and complex formation. Add ammonia, and a deep violet-blue emerges: copper-ammonia complexes are old favorites among high school chemists. In organic labs, it’s used to couple aromatic rings—a handy way to push forward complex synthesis paths. In the presence of metals such as zinc, copper chloride dips out its copper and plates it right onto the new metal’s surface; this reaction turns up in both science kits and electrochemical research. Industrially, copper chloride helps oxidize hydrocarbons under controlled conditions, paving the way for polyvinyl chloride (PVC) production and other plastics.
Synonyms & Product Names
A scientist might call it copper(II) chloride dihydrate. In catalogs, you’ll find “Cupric chloride dihydrate” or just “Copper Chloride, 2H2O.” Sometimes, import lists shorten it to “Blue copper chloride” for recognition among workers not trained in chemical nomenclature. CAS number 10125-13-0 brings it up quickly in regulatory filings. Nobody mistakes these blue-green crystals for anything else once they see the formula or hold it in hand.
Safety & Operational Standards
Health and safety matter every time you reach for copper chloride dihydrate. The compound irritates eyes and skin, so gloves and goggles become standard kit. Ingestion or inhalation of dust brings sharper problems—copper in excess causes stomach cramping, vomiting, and, if mishandled badly, liver and kidney strain. Training focuses on using fume hoods, keeping containers sealed, and washing hands after use. Response plans for spills or accidental exposure come straight from safety data sheets, and most labs store the compound in cool, dry cabinets far from food, acids, or active oxidizers. These are old lessons taught repeatedly on every shop floor and every classroom bench.
Application Area
Copper chloride dihydrate finds use in several places. Etching printed circuit boards for electronics stands out as a classic application; smooth copper patterns appear as the solution precisely removes unwanted metal. In textile dyeing, the compound brings rich blue and green hues once prized by cloth merchants. Water treatment systems dose copper chloride to knock back algae and biofouling, giving municipal water works another tool for reliable operation. Chemists value it for organic synthesis—its role as a mild oxidant or coupling agent carries through countless protocols from undergraduate labs to industrial assembly lines. Fireworks companies add copper chloride to pyrotechnic mixes for blue-green flares and sparks—the chemical rewards curiosity with brilliant colors in the night sky.
Research & Development
Laboratories still look to copper chloride dihydrate for new projects. Researchers probe its ability to act as a catalyst in green chemistry, aiming for cleaner conversion of raw materials into high-value products. Teams build sensors around its unique electrochemical properties: thin films of copper chloride respond to humidity, gases, and even light, bringing new sensing systems for environmental monitoring. Nanoparticle synthesis borrows copper salts as key starting materials; researchers create novel catalysts, antimicrobial coatings, and even biomedical probes by controlling reaction conditions around copper chloride. There’s no sense of this compound fading from research any time soon—it remains part of the toolkit for anyone chasing the next advance in chemistry or materials science.
Toxicity Research
Toxicologists give copper chloride dihydrate thorough scrutiny. Copper needed in trace amounts supports enzyme action and nerve function, but excesses bring unmistakable harm. Animal studies track effects of high copper intake—digestive upset, anemia, and liver damage top the list of concerns. Regulatory agencies set strict occupational exposure limits to keep workers safe. In the environment, copper ions linger and affect aquatic life; researchers monitor waterways near factories and recommend best practices for storage and disposal. Medical research explores ways to bind and clear excess copper from the body, protecting both workers and the broader environment from chronic exposure.
Future Prospects
Copper chloride dihydrate isn’t just a relic of past experiments. Its catalytic roles look set to expand further in sustainable industrial chemistry, where mild conditions and lower waste streams matter. Energy storage projects explore copper redox couples from batteries to solar cells, leaning on this old salt’s ability to switch oxidation states cleanly and predictably. Environmental engineering draws on its antimicrobial qualities, integrating copper-based additives into coatings that resist bacteria and prolong the life of infrastructure. Researchers track adaptations in recovery and recycling of copper compounds—hints of circular chemistry appear, where waste solutions get harvested for valuable metals rather than dumped. Demand for smart, efficient, and cost-effective materials puts this chemical right back in the conversation, driving fresh research and industrial change for years to come.
Understanding Copper Chloride Dihydrate
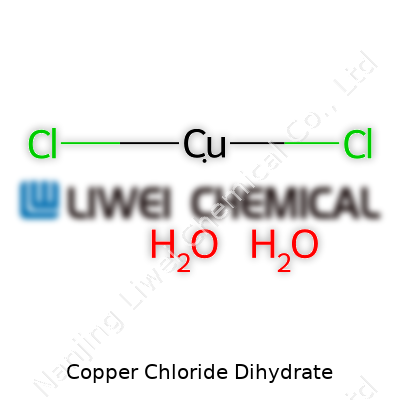
Copper Chloride Dihydrate goes by the chemical formula CuCl2·2H2O. That single formula packs in the copper ion, two chloride ions, and a pair of water molecules trapped inside the structure. This brilliant blue-green compound isn’t only an eye-catcher in chemistry labs. It pops up in plenty of practical places, from classrooms to industry floors.
Where The Formula Comes Alive
CuCl2·2H2O jumped out at me back in school, showing off its flashy color in the beaker the moment those crystals hit water. The chemical formula does more than label an obscure lab salt. It describes a specific hydrate. Take away those two water molecules, and the formula becomes CuCl2 — the anhydrous form. Toss in extra water, and you’re talking about something else. Sticking to correct formulas keeps experiments accurate and products consistent.
Why The Dihydrate Version Matters
Water matters a lot in hydrates. The water in CuCl2·2H2O isn’t just surface moisture. It’s locked into the structure. In the real world, this means anything from how much the chemical weighs to how it dissolves and reacts changes based on the formula. Techs using chemicals pay attention to water content, because two grams of CuCl2 and two grams of CuCl2·2H2O won’t give identical results in any recipe or industrial process.
From Science Class to Industry
Labs rely on the distinctions because mistakes cost time and resources. In my own chemistry days, students would watch a blue powder turn green as it lost water in heating, learning quickly that every molecule counts. Factories use Copper Chloride Dihydrate in printing, dye production, and even treating textiles. Any slip in the formula can foul up the end product or create unwanted byproducts.
Risks and Safe Practices
People working with chemicals like Copper Chloride Dihydrate need to know the risks. Swallowing or even breathing large amounts can be harmful. Always store it safely, wear gloves, and use goggles — safety data backs this up, and real accidents in labs have shown how fast a minor exposure turns into a problem. Environmental safety also matters. Copper compounds can harm fish and aquatic life if washed down drains, so safe disposal makes a difference both in schools and big plants.
Solutions for Common Mistakes
Mix-ups happen when labels get unclear or old materials lose their water to dry air. I’ve seen technicians solve that by double-checking inventory and keeping samples stored in sealed containers. Chemists in larger operations sometimes test samples for water content before big batches, using techniques like gravimetric analysis to catch mistakes early on. Education helps, too — teaching staff about formulas, the difference between hydrates and anhydrous compounds, and keeping up-to-date material safety sheets on hand.
Value Beyond the Lab
Getting the chemistry right doesn’t just affect scientists. The right formula keeps everything from school experiments to large-scale plants safe and productive. Each component, from copper to chloride to water, matters in its own way. Knowing — and using — the right chemical formula for Copper Chloride Dihydrate gives every user better results and fewer headaches down the line.
A Staple in Laboratories
Ask any chemist about copper chloride dihydrate, and there’s a good chance they’ll recall its bright blue-green crystals tucked away among their glassware. This compound shows up in many basic experiments, from teaching simple chemical reactions to helping researchers study more complex topics like electrochemistry. Students often combine it with steel wool or aluminum foil and watch the metal swap, a process called displacement. The color changes tell a story and help bring otherwise dry chemistry concepts to life.
Textile and Dyeing Industry
Textile factories count on copper chloride dihydrate for creating colors on natural fibers. This compound helps fix dyes to cotton and wool, leading to more permanent, vibrant shades. Without it, dyes may wash out too easily. The role copper chloride dihydrate plays here connects back to childhood memories of faded jeans after just a few washes—the difference is clear when dyeing goes through these proper chemical steps.
Photography Processes
Oldschool photographers, especially those into alternative process photography, sometimes reach for copper chloride dihydrate to make special effects. The compound affects silver halide crystals in photo paper or film, creating interesting color shifts or antique finishes. Black-and-white prints sometimes end up with unusual blue or green tints, all thanks to copper chemistry from chemicals like this one.
Electronics and Printed Circuit Boards
In small electronics shops and factories, copper chloride dihydrate turns up as an etchant. This means it helps cut patterns into copper sheets used in printed circuit boards. With each swipe or dip, unwanted copper dissolves away, leaving behind those intricate tracks that carry electricity through a device. Smarter and more reliable circuits owe something to how well this simple chemical helps shape and refine electronic components.
Water Treatment and Algae Control
Water plant operators sometimes use copper chloride dihydrate for algae control. Even a small amount suppresses the rapid growth of harmful algae in lakes, ponds, and reservoirs. Clearwater at a local park or a clean public fountain sometimes starts with chemical management, and copper chloride dihydrate helps with this task. Of course, dosing needs a careful hand, since copper levels that help people can sometimes pose risks to aquatic life.
Catalysts and Organic Synthesis
Chemists also pick copper chloride dihydrate when they need a catalyst—a substance that speeds up reactions without being used up itself. In processes where organic compounds come together to form pharmaceuticals or plastic components, this molecule helps reactions move along faster or more efficiently. My own work in a college lab involved watching it spark color changes and help form bonds between molecules that wouldn’t link up on their own.
The Safety Angle and Environmental Concerns
With common use comes a need for oversight. Excess copper can build up in soil and water, harming plants or fish. Simple steps like safe storage, using the right concentrations, and following local rules about chemical disposal solve most problems. Teachers, technicians, and factory workers benefit from training and clear safety rules here. Communities close to water treatment plants or textile mills also deserve transparency about what goes into their environment.
Better Practices and Moving Forward
Better record-keeping, investment in less toxic alternatives, and sharing up-to-date safety information help balance copper chloride dihydrate's benefits against its risks. Where alternatives make sense, such as in certain dyeing processes, switching over reduces worries about copper buildup. In places where this compound is essential, regular checks and careful handling keep both people and the landscape safe.
Why Safe Storage Matters
Most people never think about chemical safety until something goes wrong. Copper chloride dihydrate carries hazards, but with the right approach, accidents don’t have to happen. Whether used in a classroom, a research lab, or an industrial setting, this blue-green compound deserves respect.
Understanding the Substance
Copper chloride dihydrate is solid at room temperature and water-soluble. Breathing in its dust or getting it on your skin causes irritation. If mixed with the wrong substances or stored under damp conditions, it breaks down or becomes dangerous.
Best Practices for Storage
Here’s what keeps both people and product safe: tight sealing, a dry location, and low temperatures. A container with a screw cap made of glass or high-quality plastic works well. Even a small crack or loose lid can let in moisture, which turns the crystals to mush and ruins their quality. Granules absorb water quickly, so humidity control matters, especially in areas with muggy summers or rainy winters.
Always label the container clearly. Don’t just count on colored crystals to tell the story—labels fade and spills happen. Permanent ink holds up under basic conditions, and clear font sizes beat hand-scrawled codes any day. The label should show not just the name, but the date received or opened. This helps anyone using the chemical track shelf life and trace storage problems if a bad batch slips in.
Keep copper chloride dihydrate away from acids, reducing agents, and strong bases. This isn’t just about academic rules. Store it with something like ammonia and you risk nasty gases forming. Even in small labs, I’ve seen mixing mistakes cause unnecessary cleanups.
Controlling Access Prevents Trouble
Leaving chemicals where anyone can grab them is a recipe for trouble. Locked cabinets or dedicated chemical storage rooms put a barrier between hazardous materials and careless hands. Kids, pets, and even adults who aren’t paying attention represent a bigger threat than the chemical itself most days.
Monitoring Storage Conditions
Simple monitoring systems—hygrometers for moisture, thermometers for heat—help catch slow changes in climate that slowly ruin batches or set the stage for spills and leaks. Each year, check your supplies for crystal changes, odd smells, or clumps that signal problems. Skip the annual “spring cleaning,” and surprise emergencies turn into expensive messes.
Handling Spills and Waste
Spills should never be swept under the rug. Even a tablespoon of powder can go a long way toward making a surface hazardous. Small spills clean up with water and mild cleaning solution, but always wear gloves and keep other chemicals out of the area till it’s clear. Disposal deserves respect—a dedicated waste container, not a trash bin or sink, gives everyone peace of mind and stops local water or soil from becoming polluted.
Investing in Training
A well-trained team spots hazards before they happen. Even in basic high school labs, regular reminders go further than warning posters or five-minute lectures. Ongoing professional training makes routine hazards stay routine, not disasters.
Building a Culture of Safety
The best way to store copper chloride dihydrate isn’t a secret formula, just common sense backed by clear rules and responsible habits. With some consistency, the storage routine fades into the background, leaving more time for meaningful work and less stress about what could go wrong.
Looking Closely at the Risks
Copper chloride dihydrate pops up in chemistry labs, factories, and sometimes in school classrooms. The blue-green crystals look harmless and almost inviting. Most folks haven’t heard about the stuff outside a chemistry textbook. Still, every bottle comes stamped with hazard labels. That’s there for a reason. Touching, inhaling, or swallowing copper chloride dihydrate could mean trouble for your body. If someone ignores basic safety advice—gloves, goggles, good ventilation—they might regret it.
Copper itself can do harm. That holds true in supplements, wiring, or as a chemical compound. The chloride part doesn't make it friendlier. Even a quick search in PubChem or the CDC’s chemical database shows studies linking exposure to nausea, vomiting, and skin irritation. One time in my undergrad lab days, a friend of mine splashed copper chloride solution on her arm. The redness spread rapidly, and she had to wash it off in a hurry. The burning and that coppery color lingered for hours. Nobody walked away thinking, “That wasn’t so bad.”
How The Harm Happens
Human bodies need a small amount of copper to function. Too much flips the switch from helpful to hostile. Swallowing just a little can spark cramps, headaches, and diarrhea. Nearly every source agrees: swallowing a big amount will send you to the hospital. For skin, copper chloride can leave a mark and cause rashes. If it touches the eyes, it may sting and cause long-term damage. Breathing in the dust is bad news, even if it doesn’t cause an immediate coughing fit. Chronic exposure, especially in workspace air, drags risks for workers—liver, kidney, and respiratory harm start to show up after repeated contact.
Copper chloride dihydrate doesn’t break down in your body in a way that’s friendly or safe. The EPA treats this as a hazardous substance. A factory or school caught spilling this into the soil or water faces big cleanup costs. The water won’t simply “wash it away.” Copper build-up harms fish and aquatic plants. I grew up near a small river where a chemical spill, mostly copper-based, wiped out most of the fish for several seasons. Environmental agencies had to monitor the water for years. The lesson stuck with everyone in the area.
Fact-Based Solutions That Cut the Risk
Putting up warning signs and handing out gloves isn’t enough. Every place that uses copper chloride dihydrate needs a clear plan for storage, handling, and disposal. A locked cabinet cuts down on accidents. Good ventilation keeps the dust away from noses and lungs. Schools should keep it away from young students, and labs must never eat, drink, or store food alongside the chemical. The American Chemical Society suggests spill kits on hand and training so that accidents don’t spiral.
Disposal matters as much as safe use. Pouring copper chloride solution down the drain could wreck local waterways and lead to fines. Most cities run hazardous waste programs for proper disposal. Farms that use chemicals with copper can turn to alternative, less toxic fungicides or work under tight application guidelines.
Treating Hazards with Respect
People who use copper chloride dihydrate sometimes treat the risks as theoretical. Real-life stories, official fact sheets, and firsthand accounts prove it deserves respect. Gloves and goggles help, but a culture of safety, where training and planning come standard, makes a bigger difference. A single careless mistake takes time, money, and sometimes health to fix.
The Color and Texture Everyone Noticed in Chemistry Lab
Copper chloride dihydrate shows up in a science classroom as brilliant blue-green crystals. The color jumps out, especially when the sun hits a petri dish at just the right angle. In the palm, these crystals feel slightly cool and sometimes stick together because they pull a bit of moisture from the air. There’s a tang of sharp metal if you take a careful sniff—it’s the sort of thing that makes everyone a bit more aware of lab safety.
What’s Really Going On in That Crystallized Powder
This compound isn’t just showing off with its color. The “dihydrate” part means every unit of copper chloride holds onto two water molecules. That water is locked in, so the crystals look slightly less dry than straight-up copper salts you might find in a bottle. It isn’t just pretty—there’s purpose in the structure. With water built in, it’s a little easier to dissolve in water compared to the anhydrous version.
Solubility in the Real World: It’s Not Just Textbook Talk
Take a scoop of copper chloride dihydrate and drop it into a glass of water. You’ll see those blue-green crystals melt into the liquid fast. One key detail: it dissolves much more quickly in warm water. If you’re trying it with cold tap water, things slow down, and a few stubborn grains linger. As a kid watching this happen in class, it amazed me because the water transformed into a clear blue, almost like a magic trick.
Chemists measure solubility as roughly 69 grams per 100 milliliters of water at room temperature—much higher than a lot of salts in the cabinet at home. That number isn’t just trivia: it decides how scientists use copper chloride dihydrate in the lab. For people working with metal plating or as a catalyst in research, this rapid, almost hungry, solubility means less fuss and fewer unwanted residues.
Where Appearance and Solubility Matter
In a high school setting, this compound teaches lessons about ionic bonds, transitions metals, and what really happens to colors in water. In industry, the speed and completeness of dissolving cuts down the waiting time for mixing large batches. It’s hard to forget loading a beaker and watching the color surge through water, knowing you’d need only a stir or two to finish the job.
With its unique color, copper chloride dihydrate also flags its own presence. If anyone accidentally spills some on a bench or a white coat, the blue-green mark is immediately visible and can be washed off with plain water. Mistakes get spotted and cleaned up, which helps keep small accidents from turning into bigger headaches.
Returning to Safer and Smarter Practices
Handling any bright copper compound means washing up and staying alert. Touching copper chloride dihydrate with bare hands can irritate skin or leave a tell-tale stain. In university research, gloves and goggles were non-negotiable. Those rules came from real experiences—no one needs a chemical burn over curiosity.
Better Understanding Leads to Better Use
Every substance has its own story. Copper chloride dihydrate stands out with an appearance that invites curiosity and a dissolving power that makes it practical far beyond the chemistry classroom. Anyone working with it can appreciate its reliability, and recognizing its key traits means getting safer and more consistent results, every time.
| Names | |
| Preferred IUPAC name | Copper(II) chloride dihydrate |
| Other names |
Copper(II) chloride dihydrate
Cupric chloride dihydrate Copper dichloride dihydrate |
| Pronunciation | /ˈkɒp.ər ˈklɔː.raɪd daɪˈhaɪ.dreɪt/ |
| Identifiers | |
| CAS Number | 10125-13-0 |
| Beilstein Reference | 136873 |
| ChEBI | CHEBI:86157 |
| ChEMBL | CHEMBL1231851 |
| ChemSpider | 20571829 |
| DrugBank | DB09153 |
| ECHA InfoCard | 03c13ce5-d18a-4412-9dfa-50a1582d1d07 |
| EC Number | 231-210-2 |
| Gmelin Reference | 811262 |
| KEGG | C00245 |
| MeSH | Dichlorocupric Chloride |
| PubChem CID | 24852952 |
| RTECS number | EV3980000 |
| UNII | V9W697087M |
| UN number | UN3077 |
| Properties | |
| Chemical formula | CuCl2·2H2O |
| Molar mass | 170.48 g/mol |
| Appearance | Blue-green crystalline solid |
| Odor | Odorless |
| Density | 2.51 g/cm³ |
| Solubility in water | 75 g/100 mL (cold water) |
| log P | -1.373 |
| Acidity (pKa) | 6.4 |
| Basicity (pKb) | 6.7 (pKb) |
| Magnetic susceptibility (χ) | -62.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.925 |
| Viscosity | Viscous |
| Dipole moment | 0 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 130.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −849.0 kJ/mol |
| Pharmacology | |
| ATC code | A16AX11 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364, P501 |
| Lethal dose or concentration | LD50 oral rat: 140 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 584 mg/kg |
| NIOSH | BGMQ0002 |
| PEL (Permissible) | 1 mg/m3 |
| REL (Recommended) | 0.1 mg/m3 |
| Related compounds | |
| Related compounds |
Copper(I) chloride
Copper(II) chloride Copper(I) sulfate Copper(II) sulfate Copper(II) nitrate |

