Cobaltous Acetate: Chemical, Historical Roots, and Industry Realities
Historical Development
Cobalt, a word thrown around places like lithography labs and battery plants, only caught the scientific spotlight in the late 18th century. Back then, European chemists were separating elements that seemed inseparable. In 1735, Swedish scientist Georg Brandt proved cobalt was not just a contaminant in ores but an element on its own with rich blue coloring power. Fast forward to the 19th century, and acetate-based cobalt salts found a role in pigments for ceramics and colorants. It became vital for making glassware and porcelain shimmer with subtle hues, and this need drove small-scale manufacturing. By the 20th century, researchers scaled up cobaltous acetate for lab and industrial processes. The electric revolution and rechargeable batteries in the 21st century then pressed the compound into new forms and functions, connecting its old-world pigment role to battery science.
Product Overview
Cobaltous acetate stands as a solid with a pinkish tinge, mostly in the tetrahydrate form scientists grab for experiments and processes. This compound caught chemists’ interest since it mixes cobalt’s magnetic potential with the acetyl group’s stability. It dissolves well in water or alcohol, so it makes its way into solution-based applications without fuss. Labs stock it for precise reactivity, and battery makers use it for making cobalt-based cathodes in lithium-ion cells. Its purpose no longer stops at being a coloring agent; it now walks through doors in energy storage and complex synthesis as well.
Physical & Chemical Properties
Cobaltous acetate comes off as robust, pink crystals. Technicians describe its melting point at roughly 140 °C for the hydrated form, decomposing rather than boiling under high heat. Pop crystals into water, and it dissolves smoothly. Try it in ethanol, and you’ll get much the same solubility. Even in acetone, it finds a comfortable place. In the lab, folks pay attention to the stable Co(II) oxidation state, which holds up under usual air conditions unless hit by oxidizers. That gives it a shelf life and predictability that plenty of chemists appreciate. It weighs in with a formula of Co(CH3COO)2·4H2O on the technical data sheets, and you’ll find those four water molecules matter for both weight and reactivity.
Technical Specifications & Labeling
Every drum and jar of cobaltous acetate leaves a plant tagged with CAS number 6147-53-1 and marked with at least 99% purity for research or industrial jobs. Suppliers specify hydrate content, and regulatory paperwork always trails the package due to cobalt’s hazardous tag. The chemical labels often declare the cobalt content as a percentage; most grades sell with 23-24% cobalt. Handling instructions show up right on the package, from splash warnings to recommendations for gloves and eye protection. Most industrial buyers rely on standardized labeling in accordance with GHS (Globally Harmonized System) so that nobody in the production chain ignores the risks or the specs.
Preparation Method
Making cobaltous acetate starts with a cobalt salt—either cobalt carbonate or cobalt hydroxide. Chemists add acetic acid, and the mix reacts, bubbling as it releases carbon dioxide. Once the bubbling stops, the chemist evaporates the excess liquid and fishes out pinkish crystals as the product dries. Filtering and washing the residue clears impurities, and then lab workers dry and store it for later use. The reaction is simple, but the skill lies in controlling temperature and acidity. Too much acid or not enough heat and unwanted byproducts creep in. In battery-grade material, manufacturers follow this routine with extra steps, like recrystallization, to pull out heavy metal traces.
Chemical Reactions & Modifications
Cobaltous acetate rarely works alone for long. In catalysis labs, it teams up with other substances, helping to oxidize hydrocarbons and kickstart reactions that won’t run by themselves. Once mixed with ammonia, you get coordination complexes, which show up in dye chemistry and sensor technology. Reacting it with hydrogen peroxide, lab workers witness the formation of peroxo complexes—these are known for their bright colors and standout reactivity. Chemists often tweak its structure to tailor it for specific jobs, coaxing it into acetylacetonate or other chelate forms. This adaptability opens doors for new research, as the compound becomes both a tool and a template.
Synonyms & Product Names
In chemical marketplaces, cobaltous acetate runs under more names than some of its peers. Technicians might call it cobalt(II) acetate, cobalt diacetate, cobalt(II) ethanoate, or simply CoAc2. Older catalogues sometimes stick with its systematic name—acetic acid cobalt(II) salt tetrahydrate. Anyone handling material from global sources encounters names in German, French, or Chinese, but the character of the compound always circles back to the same pink, reactivity-handy salt.
Safety & Operational Standards
Trained workers don’t handle cobaltous acetate lightly. Direct contact irritates skin and eyes, and inhaling dust can damage lungs. Occupational standards, set by agencies like OSHA and the European Chemicals Agency, require gloves, goggles, and adequate ventilation. Plants using the substance monitor airborne cobalt levels and prevent dust clouds with closed handling. Spills get managed by wet wiping and containment, never sweeping or blowing, since cobalt dust finds its way into lungs too easily. Once the compound’s workplace run ends, it goes into sealed hazardous waste, not standard trash. No shortcuts make sense, as chronic exposure risks outweigh convenience for both people and the planet.
Application Area
Cobaltous acetate powers industrial catalysts, speeding up oxidation reactions for plastic and polymer manufacturing. Battery plants depend on it for lithium-ion cathode material; without it, the modern electric car rollouts would slow way down. In petrochemical refineries, this compound builds up key intermediates. It finds roles in dye production, giving vibrant hues stable under harsh conditions. In the world of specialized glass and ceramics, it enables unique shades that resist fading over centuries. Researchers depend on it for building complex coordination compounds and testing new applications where transition metals matter. Each industry sees it as more than a raw material—it serves as a foundation for transformation.
Research & Development
Labs never stop searching for new ways to use cobaltous acetate. Scientists push into electronics, forming nanostructured materials that improve sensors and solar cells. Some projects explore its role in organic synthesis, using it to activate carbon-hydrogen bonds, which once seemed impossible to tweak with precision. As battery research surges, teams experiment with surface-modified or doped variants to improve recharge cycles and storage capacity. The drive for green chemistry encourages researchers to reinvent its preparation, minimizing waste and avoiding harmful byproducts. Each new study builds on a mix of historical insight and high-tech curiosity, keeping cobaltous acetate in the innovation conversation.
Toxicity Research
Toxicologists keep a close eye on cobaltous acetate. Animal studies show that in high doses, cobalt disrupts heart function and triggers respiratory issues, so the chemical never enters food or drugs. Researchers map its metabolic path in the body, learning that cobalt ions bind to proteins and shift cell signaling. Recent data point to concerns about chronic exposure in industrial settings, linking extended contact to lung disease or even certain cancers. These findings drive tighter workplace regulations and the search for safer alternatives in sensitive applications. Though the research field remains active, the known risks demand steady monitoring and strong barrier methods for anyone in contact.
Future Prospects
The demand for cobaltous acetate looks set to climb as battery technologies and renewable energy storage expand around the world. Even though concerns grow about resource scarcity and ethical sourcing, recycling efforts ramp up to ease pressure on raw supply. Chemists now work on lower-toxicity variants and design new recovery methods that shrink environmental footprints. Fields like advanced catalysis, nanotechnology, and rotational molding lean into the unique qualities of cobalt acetates. The thirst for better performance and longevity ensures this compound won’t gather dust on storage shelves; its role in emerging tech keeps industries and researchers striving for breakthroughs, balancing need with responsibility.
Everyday Uses of a Uncommon Compound
Ask anyone in the street about cobaltous acetate, and you’ll probably get blank stares. In truth, this chemical crops up in far more places than people expect. My own experience tinkering with metalwork drew me into the world of metal salts, and cobaltous acetate always popped up in reference books, especially where color and electronics meet.
Color in Glass and Ceramics
Artists and chemists have leaned on cobaltous acetate for a long time when it comes to creating vivid blue tones in glass and ceramics. That rich blue in stained glass windows—think of those European cathedrals—often comes from cobalt compounds. It doesn’t take much to see that materials like this aren’t just lab curiosities. They shape art, architecture, and consumer design.
Glazing ceramics at home taught me just how touchy some pigments can be. Cobalt acetate performs steadily, providing reliable hues without bleeding or burning away in the kiln. Big companies and small studios both count on that consistency. In a world crowded with cheap colorants, knowing the history and performance of these materials helps makers minimize risk.
Batteries and High-Tech Industries
As phones and electric cars become everyday essentials, battery technology pushes into the spotlight. Cobaltous acetate forms a backbone in lithium-ion cell production, serving as a starting material for cobalt oxide cathodes. Cobalt’s role in storage chemistry is well-documented. A 2022 report from the U.S. Geological Survey highlighted how cobalt demand ties directly to growth in electric vehicle and consumer electronics sectors. Without these batteries, smartphones and EVs wouldn’t hold a charge or work as reliably.
Anyone who’s ever dealt with a failing laptop battery knows the headache of poor performance. Upgrading battery design means researching new compounds, and cobaltous acetate remains front and center in those laboratory efforts. It’s not the only option, but its compatibility and stability keep it in the supply chains, at least for now.
Catalysts and Lab Work
Large-scale manufacturing centers often use cobaltous acetate as a catalyst, especially in petrochemical industries. It speeds up chemical reactions, making processes more efficient and cutting down energy waste. In my college lab days, cobalt acetate often ended up in experiments where time or precision made all the difference. That practicality translates into real-world savings for factories producing plastics, textiles, and even pharmaceuticals. The Association of Chemical Industry reported that using cobalt catalysts trimmed costs and reduced environmental impact for dozens of member firms.
Environmental and Ethical Considerations
Behind the shimmer of glassware or the power in a lithium cell, there's a shadow—mining. Sourcing cobalt, not just cobaltous acetate, has triggered international concern around worker welfare and ecological damage. I’ve seen first-hand how small towns in resource-rich regions tend to be overshadowed and exploited. The World Economic Forum pinpointed these ethical issues, urging companies to trace their mineral supply chains and invest in better practices. Solutions include stricter export guidelines, lab-based alternatives, and better recycling programs for old electronics. We're nowhere near perfect, but more attention to these problems brings real change over time.
Looking Ahead
People rarely think about the journey from ore to finished product. Cobaltous acetate, while rarely in headlines, underpins many innovations in energy and design. Learning about these uses opens a door to smarter choices, both as consumers and as global citizens.
Working Around Cobaltous Acetate
Folks who spend time in labs or facilities producing pigments, dyes, or batteries usually come across cobaltous acetate. The pinkish compound seems harmless on first glance, but its risks run deeper than its color suggests. I’ve worked around a variety of transition metals, and cobalt stands out for its subtle but serious hazards.
What Happens to The Body
Cobaltous acetate carries health risks that deserve more attention. Breathing dust or fumes can cause coughing, wheezing, shortness of breath, and even a kind of asthma. Skin contact can result in rashes or blisters. Swallowing even small amounts causes stomach aches, vomiting, and in rare cases, organ damage. Cases of allergy are common among workers exposed over months.
The World Health Organization warns about cancer links from long-term exposure to cobalt compounds, especially in industrial settings. The International Agency for Research on Cancer has this compound in a group of substances seen as possibly carcinogenic. A study out of France tracked miners and metal workers and found higher rates of lung disease connected to cobalt. Even though everyone breathes in trace elements daily, working around larger amounts changes the equation.
Environmental Trouble
Cobaltous acetate only breaks down a little in soil and can move into groundwater if spilled or dumped. This form of cobalt reaches streams easily and poses risks for fish and wildlife. Concentrations do not stay local, often ending up much farther downstream than anyone expects. Fish exposed to cobalt experience trouble reproducing and often don’t survive nearly as long. If you live near an industrial site, this becomes a real worry.
The U.S. Environmental Protection Agency puts cobaltous compounds on hazard lists for water and soil safety. I’ve seen cases where cleanup costs skyrocket since tiny bits keep showing up long after the first spill.
Protecting People and Places
Good practices matter more than marketing claims about safety. Workers who handle cobaltous acetate need gloves and tight-fitting masks, not basic dust masks or hand sanitizer. Keeping it away from skin and eyes makes a difference. Showers and changing out of dusty work clothes before heading home also cut down on accidental exposure for families.
Companies must keep ventilation systems running smoothly and test air regularly. Real-time detectors have come a long way in catching spikes quickly. Older buildings without modern filters risk exposing workers far outside the lab or plant floor.
As someone who’s worked with chemicals for decades, responsibility falls on both management and workers. If a task seems risky, it often is, and simple steps—like ventilating a room and following spill cleanup protocols—beat relying on hopes and luck. Lab supervisors who push for regular safety refreshers tend to see fewer health complaints among their teams.
Looking Forward
More research will help us understand the full effects of cobaltous acetate, especially at low, long-term doses. Public pressure pushed companies to find safer alternatives for many chemicals over the years. Clear labeling, strict disposal rules, and ongoing education serve both workers and the wider community. Investing in safer substitutes and cleaner technology won’t erase all danger, but it keeps the conversation focused on health, not just production targets.
Understanding Cobaltous Acetate
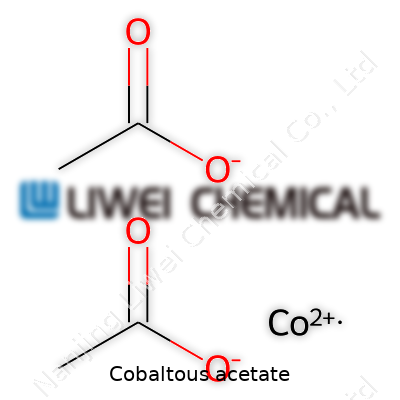
Cobaltous acetate, recognized by its chemical formula Co(C2H3O2)2, stands apart as a staple for anyone who’s dabbled in chemistry labs or worked with pigments or catalysts. That string of letters and numbers might look complicated, but it captures cobalt in its +2 oxidation state linked with two acetate groups. People sometimes refer to it as cobalt(II) acetate, signaling that +2 charge.
The Role of Simple Formulas in Real-World Chemistry
Spending any time in a science setting, you realize these formulas aren’t just trivia—they matter a great deal for safety, accurate measuring, and even making stuff like paints or rechargeable batteries perform at their best. I remember mixing solutions in university labs; skipping over that little number in a chemical formula guaranteed a poor result and extra clean-up. It counts just as much in professional research or manufacturing.
Why Cobaltous Acetate Gets People Talking
People often overlook cobalt salts unless they’re in the field, but cobaltous acetate plays into several industries. You find it in the making of deodorants and pigments. It also steps up as a catalyst, meaning it can speed up reactions without disappearing in the process. If anyone is tracking innovation, cobalt’s use in batteries makes it more relevant than ever, given the shift toward renewable energy and the big drive for electric vehicles.
Trust in the Science: Fact Matters
These details aren’t just pulled out of thin air. The formula for cobaltous acetate has support from leading chemical handbooks and peer-reviewed research. Relying on accurate formulas helps engineers, scientists, and students avoid mistakes that cost time, money, or even safety. If a process needs a precise amount of cobalt, misreading the formula can throw off the whole batch. More than once, someone has told me about projects sunk by sloppy attention to the basics.
Problems and Solutions: Sourcing Cobalt Responsibly
Cobalt’s popularity, driven by rechargeable batteries in phones and cars, raises tough questions. Mining cobalt can hurt communities and the environment if done carelessly. News reports and watchdog groups have highlighted unsafe practices in some areas. Solving this problem calls on companies to dig into their supply chain, favor sources that treat people right, and recycle whenever possible.
There’s hope: technology allows the tracking of mined minerals, and companies can now verify where their cobalt comes from. Lab research is also chasing cobalt-free alternatives or ways to reuse cobalt from old batteries. Both directions cut down on environmental problems and the risk of worker exploitation.
Everyday Chemistry: Why Details Count
Looking up the chemical formula for something like cobaltous acetate might seem tiny, but it connects to a bigger world. Accurate, factual science opens the door to safe workplaces, innovative products, and progress toward a sustainable society. Skipping those details just doesn’t cut it.
Why Cobaltous Acetate Storage Needs Attention
Cobaltous acetate pops up in more labs and factories than most realize. This chemical finds roles in dyes, catalysts, and lab experiments, but it isn’t the sort of powder you leave sitting around just anywhere. Anyone who’s dealt with compounds that can harm skin, lungs, or the environment develops a special respect for storage details.
Real World Storage Habits
Not everyone has deep shelves lined with high-tech cabinets, but the rules don’t change for cobaltous acetate. Moisture can ruin this solid, and dust particles can drift into the air. If it gets wet, changes happen, and that throws off your experiments or processes. More concerning, cobalt compounds can build up in the body with too much exposure. If you walk past open containers day after day or handle this powder without washing up, trouble follows.
Growing up with a family member who worked at a plating shop taught me plenty about chemical caution. The older workers said it best: treat every unknown drum as if it's harsher than bleach. Cobaltous acetate deserves at least as much care. Most labs keep it in sealed, labeled containers—no guessing what’s inside. Air-tight jars on shelves away from the sunlight protect both the powder and those around it. Sunlight and heat mess with chemicals in sneaky ways, sometimes making them more hazardous.
Keeping the Workplace Safe
People often store cobaltous acetate in a cool, dry space with good ventilation. Everyone jokes about 'the chemical closet,' but any program worth a dime has real rules about inventory and record-keeping. Spills and crumbling containers start as small hassles but can snowball into big ones fast.
OSHA facts back up what anyone working around chemicals learns quickly: certain cobalt compounds, including cobaltous acetate, can irritate eyes, skin, and lungs. For folks with asthma or allergies, exposure ramps up health risks. Stories from unventilated storage rooms and leaky paint cans give hard evidence: precautions matter.
Solutions Start With Good Habits
Storage improvements don’t always require big budgets. Simple steps— such as sturdy shelves or dedicated bins— prevent a lot of headaches. Farms and garages who use cobalt-based mixes for animals or metalwork can scoop powders over trays and keep unused material sealed up tight. Hand-washing routines before lunch or coffee keep toxic dust out of the break room.
Clear labeling gives everyone on the team a fighting chance if something goes wrong. Emergency showers and eyewash stations can look dramatic, but they mean something if a spill happens. One friend who took a shortcut mislabeling a blue powder regretted it; misidentified dust can cause costly cleanups or health scares, not to mention trouble with inspections.
Looking Forward: Smarter Practices
No chemical stands above basic safety. Cobaltous acetate can be stored safely when people pay attention to moisture, temperature, and airflow. Regular checks for leaks, dedicated containers, and respect for what the label says keep problems at bay. It just makes sense to care about how and where these materials wait for their next use because good storage habits protect both work and workers.
What Does Cobaltous Acetate Really Look Like?
Cobaltous acetate, known by chemists as Co(CH3COO)2, stands out in a crowd of chemicals thanks to its rich pink color. Solid samples often appear as small, rosy crystals that give off a subtle shine under the light. This pink tone makes for a handy visual cue, letting even a seasoned scientist tell Cobaltous acetate from the other powders and salts cluttering a laboratory shelf.
Feel, Smell, and Taste Are Not Welcome Here
Most folks should avoid direct contact with cobaltous acetate, and under no circumstances should anyone take a taste or a whiff. It’s toxic, and careful handling becomes non-negotiable. In my lab days, gloves and masks never felt optional. As for its feel, the salt is gritty between gloved fingers, and it clumps in humid air. Even the smallest spill leaves a visible trace, making cleanup a bit of a headache. Cobaltous acetate doesn’t give off a strong odor, which can lull newcomers into a false sense of safety—it’s still hazardous.
Solubility and Water: The Chemistry Behind the Scene
Dissolve this salt in water, and the solution turns a pleasant red-pink—a sign of the cobalt ion doing its thing. Cobaltous acetate dissolves quickly, blending with water to create a uniform solution. It also goes into ethanol and a few other organic solvents with little resistance. The solubility makes it valuable in lab work, especially for those in need of quick, even distribution when synthesizing other compounds or catalysts. Anyone working with it gets to appreciate how fast and thoroughly it can dissolve—a trait that has carried the compound beyond textbooks and into industrial and scientific use.
Stability Is a Big Deal
Storing cobaltous acetate means keeping it sealed tight. Moisture from the air grabs hold of it, and the salt soon soaks up water molecules, changing into its hydrated form. In a dry jar, the crystals last a long time. Let the humidity in and the crystalline powder becomes damp, clumpy, and less pleasant to work with. Also, cobaltous acetate holds up at room temperature but starts breaking down if things heat up much past 140°C, releasing acetic acid fumes and even forming cobalt oxide in extreme cases. Fire risk is low, but direct heat should still be avoided to protect its chemical structure and everyone’s safety.
The Real-World Impact—And a Few Words About Safety
In every application, safety takes front seat. Cobaltous acetate leans toxic, so even a small spill calls for proper disposal procedures. From my experience, the pink stain it leaves behind on glassware or benchtops always reminds technicians that this isn’t a harmless compound. Wearing gloves and working in a ventilated space are practical steps, not suggestions. In industry, limits on airborne cobalt go hand-in-hand with medical monitoring for employees who encounter this compound every day. Studies have traced cobalt exposure to lung, heart, and skin issues, prompting tighter regulations and better protective gear in workplaces.
Challenges and Smarter Practices
Cobalt extraction rates face environmental scrutiny, and companies seek ways to reclaim cobalt from used chemicals like cobaltous acetate. Safer alternatives get more attention each year, though the unique color and solubility of cobaltous acetate keep it in demand for certain types of research and industrial uses. Looking ahead, green chemistry practices—like recycling spent cobalt and substituting it in less critical processes—could limit the environmental impact. In the meantime, focusing on robust handling, airtight storage, and strong worker protections meet both safety and ethical needs. Cobaltous acetate, in all its pink crystalline glory, might always rank as a specialty chemical, but it never escapes its need for careful respect in both laboratory and industrial settings.
| Names | |
| Preferred IUPAC name | Cobalt(II) acetate |
| Other names |
Cobalt(II) acetate
Cobalt diacetate Cobaltous acetate tetrahydrate |
| Pronunciation | /koʊˈbæl.təs ˈæs.ɪˌteɪt/ |
| Identifiers | |
| CAS Number | 71-48-7 |
| Beilstein Reference | 1861050 |
| ChEBI | CHEBI:53371 |
| ChEMBL | CHEMBL1201728 |
| ChemSpider | 86419 |
| DrugBank | DB11365 |
| ECHA InfoCard | 100.013.739 |
| EC Number | 200-755-8 |
| Gmelin Reference | 1857 |
| KEGG | C02140 |
| MeSH | D003058 |
| PubChem CID | 10151 |
| RTECS number | AG3325000 |
| UNII | 7CVX6Q137F |
| UN number | UN3288 |
| Properties | |
| Chemical formula | Co(C2H3O2)2 |
| Molar mass | 177.03 g/mol |
| Appearance | Pink crystalline solid |
| Odor | Odorless |
| Density | 1.7 g/cm³ |
| Solubility in water | Very soluble |
| log P | -1.02 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | pKb: 4.1 |
| Magnetic susceptibility (χ) | +3000e-6 cm³/mol |
| Refractive index (nD) | 1.542 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -726.8 kJ/mol |
| Pharmacology | |
| ATC code | V09XX04 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H334, H341, H350, H360, H372, H410 |
| Precautionary statements | P210, P260, P264, P273, P280, P302+P352, P304+P340, P308+P313, P312, P314, P321, P332+P313, P337+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0-X |
| Autoignition temperature | 485 °C |
| Lethal dose or concentration | LD50 oral rat 120 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 660 mg/kg |
| NIOSH | AC9275000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | 50 mg/m3 |
| Related compounds | |
| Related compounds |
Cobalt(II) chloride
Cobalt(II) sulfate Cobalt(II) nitrate Nickel(II) acetate Iron(II) acetate |

