Cobalt Oxalate: From Early Synthesis to Future Potential
Historical Development
Cobalt oxalate first caught the eye of chemists during the energetic boom of 19th-century inorganic research. The growth in transition metal chemistry opened the door to discovering a class of cobalt compounds, oxalate among them, that surprised with both color and reactivity. Once researchers knew how to reliably synthesize it—crystallizing pretty pink salts from cobalt(II) solutions and oxalic acid—they began to understand a range of coordination compounds. Cobalt oxalate entered chemical manuals and found its way into pigment development, cell research, and battery precursor studies. Its route through the decades reads like a microcosm of applied chemistry, frequently revisited by teams searching for greener battery components or new catalysts. Significant patents appeared as both industrial and academic labs sought cobalt’s versatility, knowing its oxalate version had special solubility and decomposability. This foundation, supported by well-documented syntheses, keeps cobalt oxalate relevant wherever energy and stability matter.
Product Overview
Cobalt oxalate shows up most often as a powder with a soft, pink hue: sometimes rose, sometimes lavender, depending on the light and exact crystallinity. Its formula, CoC2O4·2H2O, describes the common hydrated form, which stores well and travels safely in closed containers. Manufacturers offer it for lab or industry in high purity, usually sold by the kilogram. Identification sticks to the strict labels: batch number, production date, content expressed up to four nines for trace metal work. Marketers give it nicknames: oxalic acid cobalt(2+) salt or cobaltous oxalate dihydrate, plus chemical indexes for barcode access. Not just an idle bench chemical, cobalt oxalate acts as a springboard for further cobalt chemistry—often landing a role in battery cathodes, ceramic glazes, and as catalyst material. This compound earns its spot on shortlists for lithium-ion research and ceramic projects that want genuine color stability without toxic byproducts.
Physical & Chemical Properties
A closer look at the material shows a dense, crystalline structure with a gentle shimmer upon close inspection. Cobalt oxalate dihydrate usually packs a molecular weight near 237 g/mol, and presents poor solubility in water but looks quite beautiful under moderate magnification due to its textured crystals. Density falls in the range of 2.5–2.6 g/cm3, a value that matters to process engineers scaling up material for cathodic coatings. The melting point sits above 200°C; above this level, oxalate decomposition releases carbon monoxide and carbon dioxide, changing the color as the cobalt turns into oxide forms. This variable decomposability gets exploited in calcination tasks—laboratories love the predictable breakdown for making pure cobalt oxide. Sensitivity to light and air runs low, so the compound stores for years without unwanted degradation, making inventory management simple in production-scale settings.
Technical Specifications & Labeling
Suppliers stick to tight spec lines, publishing purity at ≥99%. Impurities—iron, nickel, and copper—must stay below 0.005%. Moisture content gets tallied since the water in the dihydrate matters for subsequent reactions; often, the label clarifies whether the batch is strictly dihydrate or has mixed hydration states. Packaging always uses double-layer bags, with inner antistatic protection. Heavy-duty drums avoid both UV and static charge, and each is usually stamped with UN regulatory markings which link back to original safety data. Smart companies assign QR codes that pull up a digital CoA, giving exact traceability in multinational supply chains. The shelf life, commonly two to three years, stays dependable so long as containers aren’t breached and no temperature spike cooks off water of crystallization. These standard specs push cobalt oxalate beyond old-school academic supply, serving a decade-long battery initiative as comfortably as a one-off pigment investigation.
Preparation Method
Making cobalt oxalate on a scale starts with cobalt(II) salts—chloride or sulfate forms the base. The classic precipitation route involves slow, controlled addition of oxalic acid solution to the cobalt salt dissolved in water. Stirring the mixture at room temperature triggers an almost immediate formation of pale pink crystals, which settle as the oxalate drops out of solution. Labs filter, wash, and dry the product under vacuum or at modest heat (below 50°C), locking in the dihydrate state. On larger scales, team engineers design reactors with computerized control for precise pH and temperature, maximizing yield and keeping particle size in a narrow band—this matters during ceramic or battery precursor blending. Many research teams add a chelation step to scavenge stray iron or nickel ions, especially when medical or electronic grades are targeted. One clever approach includes nanoparticle engineering: changing agitation or seeding rates, teams can nudge crystal growth toward rods or plates, which transforms downstream properties in surprising ways.
Chemical Reactions & Modifications
Cobalt oxalate’s double life comes from how easily it rearranges its structure with heat or chemical treatment. Strong heating turns the pretty pink powder into deep black cobalt(II,III) oxide, a process central to battery cathode work. Exposing it to sodium hydroxide under reflux conditions produces cobalt hydroxide—a reaction that broadens applications for water purification and new catalyst research. In the presence of ammonium salts or phosphates, the parent molecule morphs into cobalt ammonium phosphate, which shows up in some specialty ceramic glazes. Chemists who want to shift electronic properties run it through mild reduction or complexation protocols, swapping ligands or adding surfactants to get high-surface-area materials for catalysis. These modifications respond to the push for energy-efficient processes, as every tweak can yield a superior catalyst or cheaper lithium-ion battery component. For pigment or sensor development, a bit of doping with manganese or iron transforms the absorption and reactivity profile, letting researchers tune final outcomes based on real-world need.
Synonyms & Product Names
Cobalt oxalate hides behind several names, all true to its roots. Common terms include “cobaltous oxalate,” “oxalic acid cobalt(2+) salt,” “dihydrate cobalt oxalate,” and registry-style tags such as EINECS 209-161-6. Catalogue numbers pair with CAS 814-89-3 so researchers and buyers know they have the right match, even if the trade name changes from one supplier to another. Some niche labs refer to it by a shorthand: CoOxal or Cobalt(II) oxalate. Packaging rarely flashes a brand, sticking close to chemical code to avoid mishap in busy storerooms where several metal oxalates may sit side-by-side. Transparency here keeps chemical workflows honest and accidents rare.
Safety & Operational Standards
Dealing with cobalt oxalate means treating toxicity with respect. Fine powders disperse easily, so full-face respirators, gloves, and lab coats keep users safe during handling and weighing. The compound’s dust may irritate the respiratory tract and, through skin, contribute to systemic cobalt exposure. NIOSH and European safety guidelines flag the risks, with recommended airborne concentration limits stated in material safety data sheets. Emergency showers and eyewash stations slot into any production or research space holding cobalt oxalate, and well-marked spill kits sit close at hand. Waste protocols funnel unwanted oxalate to secure collection drums, never down open drains. Waste incineration or controlled recycling ensures trace metals do not drift into municipal water or air. Proper ventilation—HEPA-grade local exhaust—fends off accidental exposure. I’ve seen firsthand that tight safety routines make all the difference, stopping minor errors from becoming lost work hours or chronic health complaints—especially in long-term battery R&D programs.
Application Area
The demand for cobalt oxalate rises and falls in step with the battery and ceramics industries. Lithium-ion innovation depends on transition metal precursors like cobalt oxalate, chosen for both cost and chemical performance. Energy storage giants seek higher-capacity, longer-life cathodes, where precursor purity makes or breaks test results. The ceramic sector values cobalt oxalate as a route to colored glazes and stable oxide layers, offering reliable hue with less risk of toxic byproducts when compared to chromium-based colors. Catalyst development leans on the material to test out new reactor designs—here, surface area and decomposition temperature drive catalyst efficiency. Research teams exploring wastewater remediation investigate oxalate-based filters, hoping that smart modification can grab heavy metals from contaminated streams. In pigments, artists and mass-market paint suppliers tap the reproducible color stability, which works in both high- and low-fire processes. Every sector I’ve worked with wants repeatability, and cobalt oxalate delivers that so long as specs stay tight and handling follows protocol.
Research & Development
Frontline research stretches from nanotechnology to environmental science. Battery groups test cobalt oxalate as an intermediate for next-gen cathode crystals, comparing it with other salts in terms of energy density, thermal stability, and lifecycle cost. Materials chemists invest effort in downsizing oxalate particles, aiming for new morphologies that boost conductivity or change catalytic profiles. There’s a groundswell of work on hybrid composites: teams infuse carbon networks with cobalt oxalate, chasing higher supercapacitor performance that could someday outperform existing systems. Water researchers tackle sequestration strategies, modifying oxalate structure to capture or destroy pollutants in ways traditional filters cannot. Pigment chemists revisit cobalt oxalate as regulators clamp down on heavy metal releases; the aim is safer, greener blue and violet hues in glass or enamel. I’ve collaborated on a few grant proposals where the synthesis and post-treatment of cobalt oxalate determined the project’s success on both technical and environmental fronts, especially as recycling enters the discussion.
Toxicity Research
Cobalt’s double-edged nature—essential trace metal but also a risk at high exposure—drives much of the attention on oxalate toxicity. Animal studies show that excessive inhalation or ingestion can disrupt heart, thyroid, and red blood cell health. Regulatory agencies set strict limits; for instance, permissible air concentrations run in the microgram range. Chronic exposure can produce dermatological and respiratory symptoms, reinforcing the need for closed handling and monitored workspace. Toxicological teams in Europe and the Americas run long-term studies on both acute and chronic effects, using findings to push for better personal protection equipment and improved workplace monitoring. Waste disposal guidelines mirror the lessons of old: landfills and open drains must stay cobalt-free. Medical researchers hold out hope that understanding oxalate pathways could inform better monitoring in occupational settings, closing the loop between industrial chemistry and public health. In my own work I’ve seen the value of double-checking every batch before release—not just to hit regulations, but to keep the lab team healthy over the long run.
Future Prospects
The next decade brings a real test for cobalt oxalate. With battery recycling picking up speed and energy storage shifting toward renewables, demand for stabilized cobalt intermediates will climb in parallel with raw cobalt sourcing challenges. Companies invest steadily in greener synthesis pathways, hoping to cut environmental impact while boosting product yield. Nanostructuring stands ready to change how oxalate-based materials perform—smaller, smarter particles promise increased catalytic rates and energy density for future grids. Eco-labeling efforts tie cobalt oxalate’s reputation to lower-waste synthesis and closed-loop life cycles: customers want the traceability and responsibility that goes beyond paperwork. Regulatory pressure, both for worker safety and environmental protection, encourages safer packaging and more transparent sourcing of cobalt. As I see it, the race isn’t just toward better batteries or pigments, but toward a chemistry culture where high performance and minimized hazard go hand in hand. The evolution of cobalt oxalate will reflect how the industry meets the push for both excellence and responsibility.
Understanding What Cobalt Oxalate Brings to the Table
In the world of specialty chemicals, cobalt oxalate doesn’t grab headlines, but it quietly carries serious weight in technology, manufacturing, and green energy. This powdery compound blends science with function, helping drive forward processes that reach into batteries, pigments, and high-strength alloys. Its pinkish tint hints at cobalt inside, but the real story lies in the applications forged wherever folks push materials to work harder or last longer.
Batteries: Building the Next Generation of Power
If you keep an eye on electric vehicle news, you probably catch glimpses of cobalt talk—often around supply chain or ethical sourcing. Cobalt oxalate steps in behind the scenes. Technicians use it to prepare battery-grade cobalt powders and other compounds vital for lithium-ion batteries. These batteries rest inside everything from cell phones to electric cars. Without cobalt’s chemical properties, batteries tend to lose charge more quickly and don’t last as long. By refining cobalt through its oxalate form, battery makers gain the controlled purity and fine particle size that today’s demanding devices need.
This isn’t just a matter of gadget performance. As governments push clean energy goals, cobalt compounds remain critical for grid storage and electric vehicle reliability. Researchers wrestle with finding alternatives, but current cathode chemistry still relies on well-prepared cobalt to keep supply worries in check while maximizing performance. Knowing where each compound comes from, and ensuring materials are responsibly sourced, becomes as important as the chemistry itself.
Ceramics and Glass: The Color and Strength Factor
Artists and industrial chemists both value color, though for different reasons. Cobalt oxalate converts into cobalt oxide under heat, and that oxide gives an intense blue color to pottery glazes, tiles, and certain types of specialty glass. That rich blue has signaled “quality” in ceramics for centuries, but the manufacturing process trades on chemistry that’s still being refined.
I once toured a ceramics lab where a technician showed how tiny differences in the oxalate’s structure could swing the shade from sky blue to deep navy. Beyond color, this same cobalt also boosts durability in glass for electronics or laboratory gear—the stuff that gets beat up or faces sudden temperature changes.
The Catalyst Game: Cleaner Air, Better Chemicals
Cobalt oxalate finds another home inside catalysts, the silent partners in both cleaner emissions and chemical synthesis. Take a look at what comes out of a car or power plant; catalysts with cobalt roots help break down pollutants before they hit the air. Chemical plants use these catalysts to drive reactions that build everything from plastics to medicine. Small tweaks in the oxalate’s preparation set up catalysts that work faster, produce fewer byproducts, and waste less energy.
What Lies Ahead: Transparency, Responsible Sourcing, and Smarter Chemistry
Global demand for cobalt-based products isn’t shrinking. Yet, this demand brings questions about mining impact, labor standards, and recycling. Smart buyers search for suppliers who trace their chain of custody, looking out for both the environment and local communities. Researchers aim to stretch every gram further by recycling old batteries and using synthetic chemistry to trim waste during manufacturing. By staying open about sourcing and innovating with production methods, industries can keep benefiting from cobalt oxalate’s unique properties without turning a blind eye to bigger responsibilities.
The Formula and Its Meaning
Chemists look at Cobalt Oxalate and write it as CoC2O4. This formula signals a blend between cobalt, a shiny transition metal, and oxalate, a compound made from carbon and oxygen. On the surface, these six characters tell us how many atoms snap together to make one molecule. In practice, it’s a doorway into an entire world of color, reactivity, and possibility.
Bringing Science Closer to the Real World
Plenty of people cross paths with cobalt oxalate without knowing it. It shows up in pigment manufacture, battery technology, and often as a chemical middleman in factories making things people use all the time. In my early days in the lab, Cobalt Oxalate would arrive as this lavender powder in jars with tight lids. It stained your gloves, stuck to glass, announced its presence in a way that more common salts never did. That color—pale but distinctive—makes it a popular choice for artists’ pigments. For folks working with lithium-ion batteries, Cobalt Oxalate acts as a step on the road to producing cobalt oxide, a vital material inside the rechargeable batteries powering phones and electric cars.
Safety, Environment, and Responsibility
Every chemical requires a thoughtful approach. Cobalt Oxalate can be toxic if mishandled. Touching or breathing its dust always raised alerts with our safety officer; it isn’t something you sweep off the bench at the end of a shift. The environmental impact of cobalt mining, which supplies much of the world’s cobalt, tells a bigger story. Open-pit mining scars landscapes, rivers fill up with metal run-off, entire communities face health challenges. All of this sits behind each gram of Cobalt Oxalate that chemists use. As a user and teacher, I understand these connections and keep looking for cleaner methods—think recycling electronics for cobalt, switching to less harmful starting materials, choosing suppliers that meet good ethical standards. Lately, some manufacturers have set up closed-loop systems, recovering cobalt from spent batteries, keeping more out of mines and back into useful products.
Why the Formula Matters in the Field
Knowing the formula CoC2O4 does more than solve a test question. It lets scientists and producers predict how the compound will react. Drop it in strong acid, and you get a fizz of carbon dioxide; heat it, and it breaks down to give cobalt oxide and gas. Understanding these reactions isn’t academic. Battery plants need precise timing and temperatures to make cobalt oxide that actually works; artists want colors that last. I learned quickly that swapping even one element in the formula could ruin a batch or spark a new discovery. One colleague managed to “dope” cobalt oxalate with other metals, opening up bright new colors for ceramic glazes. Sometimes, a formula serves as a starting point for creativity or innovation.
Opportunities for Progress
Science never stands still. Researchers keep chipping away at the problems linked to cobalt and oxalate use. I’ve seen universities work on battery chemistries that use less cobalt, or aim for safer cobalt compounds that don’t shed dust so easily. Better recycling tracks cobalt from old phones back into new batteries. Chemistry teachers use simple formulas like CoC2O4 to get students thinking about where elements come from, and what their use costs the planet. By connecting classroom learning and real-world impact, we spark curiosity and a bit of healthy caution too. In my own work, making careful choices became a habit that stuck. Cobalt Oxalate may look simple on paper, but its story goes far beyond the laboratory counter.
What is Cobalt Oxalate?
Cobalt oxalate shows up in quite a few niche labs. Folks working in pigment manufacturing, battery research, or advanced ceramics sometimes work with this pink-to-red powder. Chemists like it for its role as a catalyst or precursor to other cobalt compounds. Most people outside these circles won’t bump into cobalt oxalate, but questions about its risks deserve a real answer.
The Trouble with Cobalt Compounds
From a young age, I learned to watch out for dusts and fine powders in any lab job. Cobalt, in any form, is not a benign element. It springs up in health conversations because the human body needs tiny amounts of cobalt as part of vitamin B12. The story changes dramatically when exposure moves past trace nutrients or gets repeated.
Inhaling airborne cobalt compounds leads to respiratory irritation and – in serious scenarios – can scar lung tissue. The International Agency for Research on Cancer classifies many cobalt compounds as possible carcinogens. Animal studies suggest long-term exposure, especially by inhalation, equals a higher risk for cancer. There are reports of factory workers who developed asthma, cough, or sensitization from inhaling cobalt dusts.
Cobalt oxalate also comes with hazards tied to oxalates. Swallowing it or letting it sit on your skin is a mistake. Oxalates are notorious for causing kidney stones and can irritate the digestive tract. Some folk can’t help their curiosity or may forget PPE just once. Swallowing even moderate doses can result in nausea, vomiting, and stomach pain.
How Much Exposure Is Too Much?
It can be tough to estimate what “too much” looks like. Workplace exposure limits vary by country. For example, the Occupational Safety and Health Administration (OSHA) sets an exposure limit for cobalt compounds at 0.1 mg/m3 averaged over an eight-hour shift. Most consumer goods don’t use cobalt oxalate, so general public exposure is not worth worrying about, but workers must remember the risks.
Personal protection matters a lot. Lab workers – myself included – rely on gloves and dust masks anytime we handle cobalt salts. I learned early on that a careless gesture can turn a regular shift into a near-miss. Gloves and goggles are non-negotiable. Good ventilation kicks risk down another notch. Properly labeled storage stops accidents from happening in the first place.
Safer Handling: Moving from Concern to Practice
Proper training can save people from a world of hurt. In my experience, the best labs run regular hazardous materials refreshers and make spill kits easy to access. I’ve seen companies move to pre-weighed, sealed packets for toxic powders. This simple tweak limits airborne exposure and makes cleanup easier.
Getting rid of cobalt oxalate waste must be handled by hazardous chemical procedures. Never down the sink. Waste contractors who follow environmental laws keep cobalt out of waterways, protecting wildlife and people alike. Rushing disposal never works out.
Alternatives and Looking Forward
The battery industry, for example, searches for cobalt-free alternatives every year. Some new lithium technologies focus on iron or manganese, both less toxic. If more manufacturers switch to these, the risks from cobalt handling will shrink.
Cobalt oxalate is not a casual lab reagent. Safety training, reliable PPE, and good habits stop most mishaps. Society can reduce the hazards further by supporting research into cobalt substitutes and enforcing tough workplace safety laws. The best outcome keeps workers healthy and chemical risks far from daily life.
Real World Settings Demand Respect for Risks
Anyone working around chemicals understands that routine turns dangerous if shortcuts sneak in. Cobalt oxalate isn’t just a technical label; it’s a powder with baggage. Researchers and industry experts know its toxicity can harm lungs, the environment, and anyone unprepared for an accident. I remember the first time a close colleague handled it without a mask. She coughed, eyes watering—one small cloud was enough. That’s the reality for folks handling this substance in labs or warehouses. Throwing a sack on a dusty shelf simply won’t cut it.
Good Storage = Fewer Accidents
You want science-backed habits, not wishful thinking. The basics kick off with a single word: isolation. Cobalt oxalate can’t share space with food, personal items, or anything that could spark a chain reaction—especially acids or combustible materials. A locked chemical storage cabinet helps. Keep it cool, dry, and out of direct sunlight. The danger increases if the powder gets damp or if someone unfamiliar tries a shortcut with labeling. Humidity hastens slow decomposition, which releases harmful gases. Simple desiccant packets in sealed containers help control that, and anyone running a small operation in a humid climate knows how fast moisture creeps up.
Labels and Training Make All the Difference
Labels matter. Not fancy, but clear. I’ve seen too many bottles marked with faded ink or a sticky note. In one shared lab, a faded label nearly caused a mishap because someone grabbed the wrong jar, thinking it was copper sulfate. Training means pointing out these specifics, not just telling folks to “be careful.” If the compound’s going to stay on site for any length, periodic checks show if the container seals properly or if powder has escaped. That’s just practical.
Equipment and Exposure Control
Keeping cobalt oxalate locked away helps, but it doesn’t stop accidents by itself. The next big piece is personal protection—gloves, lab coats, goggles, real respirators. After handling, anything that touches the powder should get cleaned right away. Even seemingly tiny bits cause problems if they wind up on desks, doorknobs, or food prep areas. This doesn’t just show up in textbooks; it shows up in real health records when occupational exposure builds up over weeks and months. Studies link cobalt exposure to lung problems and skin irritation, and in big enough quantities, more serious effects on the heart and thyroid.
Thinking about Waste and Emergency Prep
Any facility using cobalt oxalate has responsibilities extending past daily storage. Spill kits, including absorbent material and protective gear, should be nearby—preferably in every room where the chemical shows up. Clean up small spills quickly, scoop up solids, and ventilate the space. Never toss leftovers in the trash. Work with hazardous waste companies who know the disposal process, not only for legal reasons but because groundwater contamination changes lives far beyond the workplace.
Solutions Rely on Habits, Not Just Rules
Clear written protocols go a long way, but real safety happens because people care about one another. Talk through the “why,” not just the “how.” Make habits visible: someone checking humidity indicators, logging usage, or swapping out cracked gloves before a problem happens. That culture of care flips chemicals from random hazard to something manageable, even for workers who haven’t seen the inside of a university lab.
Getting Up Close with Cobalt Oxalate
Cobalt oxalate pops up often in labs working with transition metals or studying battery materials. Its look catches the eye right away. You see a pinkish powder, sometimes leaning toward a salmon hue, and not in the shining way that crystals like quartz offer. It feels a bit coarse between the fingers, almost sandy. The color comes from cobalt ions tangled inside the lattice with oxalate, and this isn’t some random tint. That color signals something about its structure, hinting at hydration and oxidation states that chemists learn to spot with a glance.
Anyone who has opened a sample bottle or tried to weigh it notes the way it clumps, reluctant to spill smoothly from the weigh boat. It doesn’t glisten under the light, but the distinctive shade provides a quick tool for checking purity. You won’t run into clear, colorless, or shiny versions. If you do, something’s likely wrong with your batch.
Solubility: Straightforward but Problematic
You put cobalt oxalate in water hoping for a fast dissolve, and you’ll wait a long time. This compound barely goes into solution. In cold water, the powder mostly settles to the bottom. Even stirring with a magnetic bar for an hour won’t change much—the water barely tinges with color. This poor solubility hiccups across science from chemical synthesis to waste treatment. People working on recycling cobalt from spent lithium-ion batteries run into this snag almost right away. If cobalt oxalate refuses to budge, getting that cobalt back takes extra steps and more chemicals. This doesn’t just drain resources; it stretches out turnaround times and means more cost.
I remember trying to recover cobalt from spent catalysts in one of my old projects. Cobalt oxalate would form at the wrong step and stick to the glassware, refusing to dissolve with water or mild acids. I had to reach for stronger acids and heat, eating into time and safety margins. Others I know working with recovery plants have echoed the same pains. Its slight solubility in acids like hydrochloric or sulfuric gets used on a large scale, but these acids come with their own dangers and waste issues.
Why This Even Matters
Cobalt is vital for making rechargeable batteries, especially those driving smartphones and electric vehicles. Extracting cobalt efficiently keeps battery prices in check and ensures enough material lands in the right places. When cobalt oxalate clogs up the process due to low solubility, it slows the shift to cleaner technology, costs users extra, and adds to environmental challenges. From mining through recycling, every bump in the road lands back on the companies and communities relying on affordable, sustainable technology
Moving Forward
Researchers keep looking for ways to coax cobalt out without so much hassle. Some folks try chelating agents or smart solvents that can grab hold of the cobalt ions without needing punishingly strong acids. There’s interest in high-pressure or microwave techniques that break down stubborn crystals faster. Training new chemists to recognize the headaches and turn to alternative recovery routes early in a project can save time, materials, and headaches. More labs now share small breakthroughs with open-access data, letting everyone stuck on cobalt oxalate’s behavior get a leg up. Promoting that kind of teamwork and finding safer, more efficient ways to recover cobalt could close the loop on battery lifecycles, reducing the pressure to dig up more raw material
| Names | |
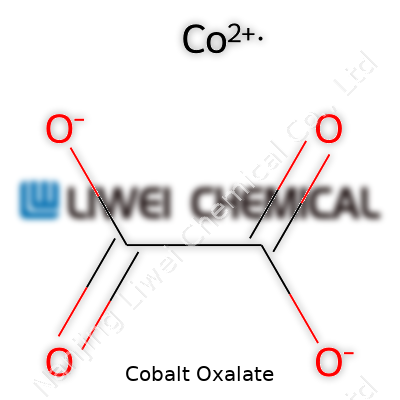
| Preferred IUPAC name | Cobalt(2+) ethanedioate |
| Other names |
Cobaltous oxalate
Cobalt(II) oxalate Ethanedioic acid, cobalt(2+) salt (1:1) Cobalt(2+) ethanedioate Oxalic acid cobalt(II) salt |
| Pronunciation | /ˈkoʊ.bælt ˈɒk.sə.leɪt/ |
| Identifiers | |
| CAS Number | 814-89-5 |
| Beilstein Reference | 35864 |
| ChEBI | CHEBI:84949 |
| ChEMBL | CHEMBL1201104 |
| ChemSpider | 15238 |
| DrugBank | DB14557 |
| ECHA InfoCard | 22b72e55-4cf0-4e37-814b-d553d2c72b43 |
| EC Number | 208-090-8 |
| Gmelin Reference | 58455 |
| KEGG | C18635 |
| MeSH | Cobalt Oxalates |
| PubChem CID | 101782 |
| RTECS number | GN1225000 |
| UNII | 07JH43P670 |
| UN number | UN3288 |
| CompTox Dashboard (EPA) | DHV58DI326 |
| Properties | |
| Chemical formula | CoC2O4 |
| Molar mass | 146.94 g/mol |
| Appearance | Light pink powder |
| Odor | Odorless |
| Density | 2.58 g/cm³ |
| Solubility in water | Insoluble |
| log P | -2.76 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.4 |
| Basicity (pKb) | 7.3 |
| Magnetic susceptibility (χ) | +3500e-6 cm³/mol |
| Refractive index (nD) | 1.736 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 93.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -826.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -644.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, suspected of causing cancer. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H334, H335, H350, H410 |
| Precautionary statements | P261, P264, P270, P272, P280, P302+P352, P304+P340, P308+P313, P312, P314, P321, P332+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | NFPA 704: 1-2-0 |
| Autoignition temperature | > 535 °C |
| Lethal dose or concentration | LD50 oral rat 750 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >2000 mg/kg |
| NIOSH | WL36750 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 20 mg/L |
| IDLH (Immediate danger) | 250 mg Co/m3 |
| Related compounds | |
| Related compounds |
Cobalt(II) sulfate
Cobalt(II) chloride Nickel oxalate Iron(II) oxalate Cobalt(II) carbonate |

