Cobalt Chloride Dihydrate: From its Discovery to Modern-Day Uses
Historical Development
Decades ago, cobalt chloride drew the interest of early chemists, not because of a grand discovery, but rather through practical mineral extraction. Georg Brandt separated cobalt as a metal in the early 18th century, but it didn’t take long for cobalt compounds to appear in laboratories across Europe. Cobalt chloride, in its dihydrate form, slipped seamlessly into the world of color science and analysis. Chemists quickly noticed its shifting shades in response to water—blue when dry, pink when hydrated—making it a favorite for humidity detection long before digital sensors entered the scene. Its history remains tied to a long tradition of analytical chemistry and glassmaking, echoing the broader history of inorganic salts and their experimental value in both academic and industrial circles.
Product Overview
Today, cobalt chloride dihydrate surfaces in the chemical industry, education, and scientific research. It arrives with a distinctive pink hue, ready to be transformed either by temperature change or exposure to air. Sometimes referred to as cobaltous chloride dihydrate, this compound features two water molecules for every cobalt ion. That hydration level shapes its shelf life, risk profile, and usefulness in laboratory settings. In bottles lined up in classrooms and research labs, it teaches concepts like chemical equilibrium and crystallization, sliding easily into both starter chemistry sets and technical industrial supplies.
Physical & Chemical Properties
Cobalt chloride dihydrate comes as reddish-pink crystalline solids that dissolve readily in water and alcohol. Its chemical formula stands as CoCl2·2H2O. These crystals turn blue after losing water—an unmistakable transformation for anyone who’s watched a cobalt-based Humidity indicator dot dry out on a silica gel packet. The hydrated and anhydrous states depend on temperature and atmosphere, a characteristic that guides its practical applications. The dihydrate’s melting point hovers around 86°C. In daily handling, the powder’s tendency to clump and rapidly wick moisture from the air provides its own kind of visual drama, making proper storage a constant subject in labs.
Technical Specifications & Labeling
Labels on bottles usually show purity percentages, batch numbers, and hazard symbols. Reputable suppliers tag the product with information about trace metals or possible contaminants, supporting traceability and compliance with global regulations. You find detailed safety statements, precise molecular weights (about 165.87 g/mol), and color indicators for quick identification. For most laboratory and industrial users, up-to-date labeling means knowing what risks and regulatory burdens accompany each shipment—a necessity, especially after tightening safety standards targeting heavy metals.
Preparation Method
Manufacturers start by reacting cobalt (II) oxide or carbonate with hydrochloric acid in water, a straightforward process rooted in centuries-old reaction chemistry. This yields cobalt chloride in solution, which then cools and crystallizes as the dihydrate form. Removing excess water at controlled temperatures assures the two-water formula. Since atmospheric moisture can shift the hydration level, packaging right after crystallization matters. Working in the chemical industry, I’ve watched multi-ton reactors churn out pastel pink slurries, destined for dryers and sealed containers—a reminder of how scale transforms lab reactions into global supply chains.
Chemical Reactions & Modifications
Cobalt chloride dihydrate showcases rich chemistry. Heating drives off water, revealing blue anhydrous cobalt chloride. If you reverse the process and let the blue powder reabsorb water from the air, the familiar pink hue returns. Drop the compound into ammonia or strong alkali and you get a range of colored complexes—a spectrum that’s grabbed the imagination of generations of chemists. Students and researchers often deploy it as a precursor for other cobalt salts, adding acids, bases, or other metal ions to prompt a cascade of reactions. These transformations make the compound a flexible building block in both teaching and more advanced synthesis.
Synonyms & Product Names
Names like cobaltous chloride, dichlorocobalt, and CoCl2·2H2O each direct users to the same pink salt. Specialty suppliers sometimes brand it with internal codes or grade-specific names, separated only by regulatory distinctions or purity. This cluster of names can cause confusion in procurement, especially when industries with different traditions—thinking pigments versus battery manufacturing—cross paths. It’s a good practice to double-check molecular formulas and water content, since dropping the “dihydrate” tag can mean big shifts in behavior and toxicity.
Safety & Operational Standards
Cobalt chloride dihydrate deserves careful handling. It carries carcinogen recognition in many regulatory regimes. Safety Data Sheets detail the need for gloves, eye protection, and fume hoods. Proper user training, regular monitoring, and containment practices lower exposure risks, a point that comes up often in training sessions for lab techs and students. Disposal needs to follow hazardous waste protocols, since cobalt and chloride ions both feature in environmental monitoring checklists. Part of keeping a healthy workplace means focusing as much on risk communication as on chemical knowledge.
Application Area
Beyond indicator cards and chemical demonstrations, cobalt chloride dihydrate turns up in battery manufacturing, pigment production, and research on coordination compounds. It supports biological laboratories investigating cellular uptake of metals and plays a part in dye formulation for glass and ceramics. Anyone working in electronic component manufacturing comes across it as a trace element additive. For years it formed the backbone of basic chemical education, its color shifts offering visible proof that atoms and molecules never stop moving, even when our eyes tell us otherwise.
Research & Development
Research labs keep cobalt chloride dihydrate on hand for new materials development. Chemists looking at battery cathodes screen it alongside other cobalt salts. Medical research teams test its effects on living cells, looking for insights into both toxicity and essential trace functions. My own early work involved using it as a humidity marker for reactions where water content proved crucial, a trick passed down from supervisors who learned the ropes from their mentors decades before. The compound remains part of broader efforts to refine next-generation sensors, recyclable materials, and diagnostic reagents.
Toxicity Research
Studies point to cobalt chloride dihydrate as both a toxic and possibly carcinogenic compound, with risks rising during prolonged or high-exposure scenarios. Inhalation or skin contact brings risks of dermatitis and respiratory irritation, while chronic exposure links to more serious outcomes like heart problems and thyroid impacts. Outreach to workers and students continues, nudging everyone to treat this familiar compound with respect. Toxicity tests on lab animals and cell cultures provide guidance for regulatory agencies, drawing a line between safe experimental use and unacceptable chronic exposure. Debates about safe thresholds continue, informed by a growing body of occupational studies.
Future Prospects
Looking forward, demand for cobalt chloride dihydrate seems tied to expanding battery technologies, advanced sensors, and greener pigment preparations. Research pushes for safer handling methods, less wasteful production cycles, and improved recovery from spent products. There’s strong momentum around finding cobalt alternatives in batteries, but as that science matures, cobalt salts will likely keep serving as benchmarks for stability and performance. The compound might fade from some traditional sectors as alternatives move in, but its role in analytical chemistry and basic teaching appears steady, tracing a long arc through the evolving landscape of chemical practice.
A Splash of Science in Everyday Life
Most people haven’t handled cobalt chloride dihydrate, yet its pink crystals hold an important place in science and industry. If you ever walked into a high school chemistry class, you might have spotted strips of cobalt chloride paper. These pink strips turn blue after soaking up moisture, giving students a hands-on way to see humidity in the air. It’s a classic experiment, and after years of rain-watching in different corners of the world, I’ve learned that this little trick offers a simple lesson: sometimes, the smallest chemicals open doors to understanding the environment around us.
Testing and Tracking Water
Factories use cobalt chloride dihydrate in desiccant packs to track moisture. That bright pink color signals dryness, while a shift toward blue means water’s sneaking in. In my own experience working with storage solutions, replacing silica gel desiccants with those treated with cobalt chloride brought peace of mind; it’s far easier to trust a color signal than guess at a printed expiration date. Simple, visual feedback like this helps companies protect sensitive products like electronics and pharmaceuticals.
Behind the Lab Bench: Chemistry’s Hidden Hero
Besides moisture detection, this compound keeps chemists busy. In the lab, it serves as a source of cobalt ions for making other materials, especially catalysts used in research. I remember finally getting a stubborn reaction to work after swapping in cobalt chloride—the change felt small at first, but it cut hours off the process and reduced chemicals lost to waste. Reliable reagents matter more than most folks realize, especially for people hoping to save costs and avoid safety headaches.
Art and Color in Unexpected Places
Cobalt chloride dihydrate leans into a bit of showmanship with its shifting colors. Some artists dig into this property and use the salt in specialty inks or paints that change with humidity. Beyond novelty, it echoes an old-world fascination with color that stretches back through centuries of pigments and dyes. For textile hobbyists who want a dash of interactivity, this chemical delivers a kind of magic rooted in science.
Health and Environmental Caution
Cobalt compounds raise questions about safety. Prolonged exposure—breathing powder or getting skin contact over time—can trigger allergic reactions or pose health risks. Personally, I’ve seen workplaces grow more cautious over the years, moving to better labeling and stricter safety protocols once the risks became widely known. As science sheds more light on the long-term impacts of such materials, there’s a push for alternatives or proper containment, so we protect people and the environment.
Looking Forward
Anyone curious about common chemicals might look past cobalt chloride dihydrate, but digging deeper reveals a world of practical uses. Whether tucked into a weather-detection kit, driving a breakthrough in the lab, or adding surprises to art, this powder keeps turning up in unexpected places. Safer handling and thoughtful disposal will only make its role more effective in the future.
Cobalt Compounds and Everyday Risk
Cobalt chloride dihydrate comes up in many discussions about chemical safety for a reason. This vivid blue or pink salt catches the eye and crops up in science classrooms, humidity sensors, and even some hobby projects. People see it and assume it’s harmless, especially since it appears so often in non-industrial settings. But the bright color hides a more complicated story for anyone who isn’t careful.
What Science Tells Us
Exposure to cobalt compounds presents real health concerns. Scientific studies link inhaling cobalt dust or fumes to lung irritation, asthma-like symptoms, and a higher chance of developing chronic respiratory diseases. Skin contact can cause rashes and allergic reactions. These aren’t only minor irritations; cobalt’s reputation as a sensitizer means repeated handling ramps up the chances of the body reacting badly over time.
More recent research raises eyebrows for stronger reasons. A European Chemical Agency (ECHA) report classified cobalt chloride as a substance of very high concern due to its potential carcinogenicity and reproductive hazard. Lab tests on animals show harmful impacts on fertility and development. Workers facing cobalt dust in industrial settings see greater health risks, but casual use at home still raises exposure concerns.
I worked with cobalt chloride in a college lab and saw classmates brush off warnings, often handling it with bare hands. One student ended up with cracked, red skin; another coughed for weeks after spilling powder on a benchtop. Most shrugged these incidents off, not linking them to the cobalt until an instructor explained the risk, citing studies from the National Institute for Occupational Safety and Health (NIOSH). That moment left a lasting impression.
Everyday Exposure: Underestimated or Overblown?
People often underestimate the risk, thinking, “It’s just for experiments, a few drops can’t hurt.” The trouble creeps in with chronic, low-level exposure. Even hobbyists at home, without the proper ventilation or personal protective equipment, can face the same health threats highlighted in industrial safety manuals. Ingesting cobalt salts, breathing in dust, and even unwashed hands touching food add up. For anyone with a history of asthma or allergies, the risk only goes up.
Reducing the Risk: Simple Habits, Big Benefits
Institutions and industries have strict standards for handling cobalt compounds, but those rules count at home too. Practical steps work: gloves, goggles, and a sturdy fume hood make a world of difference. Good habits—washing hands, labeling containers, and cleaning surfaces—reduce accidental exposure.
Disposal needs attention. Pouring leftovers down the drain pollutes water, harming both humans and wildlife. Local hazardous waste programs offer safer alternatives for getting rid of unused cobalt chloride.
Some schools now revisit their policies, limiting student access to cobalt chloride and switching to safer alternatives in chemistry sets. Education about real-world risks leads to smarter choices and fewer sharp lessons learned the hard way.
The Case for Caution
Commonplace use of cobalt chloride dihydrate hides the reality around its hazards. People deserve straight answers about what this substance does to the body. Simple steps protect families, students, and workers alike. Safety starts with the facts, not just a warning label. Relying on up-to-date research beats old habits, and everyone stays healthier for it.
Why Care About How We Store Chemicals?
Years of work in lab settings have shown me that safety often comes down to small choices, like how we keep even simple supplies on the shelf. Cobalt chloride dihydrate is one of those substances that won’t catch anyone’s eye at first glance—a dusty blue or purple crystalline powder, about as threatening as table salt. But under the surface, it brings some real risks that deserve respect. Mistakes or shortcuts in storing it have led to accidents, some only a little scary, others ending in emergency room trips and regulatory fines.
Understanding Cobalt Chloride Dihydrate’s Risks
Cobalt chloride dihydrate can cause health problems by skin contact, inhalation, or ingestion. I learned quickly in lab training that even small exposures make people feel sick; too much over the years can even raise cancer risks. It also reacts to changes in humidity, showing color changes as it pulls water from the air—a neat chemistry trick, but a warning sign, too. If the container gets left open, this chemical can clump up or break down, making measurements less reliable and handling more dangerous. This is far more than a paperwork problem. During one hot summer, I saw a batch ruined just because someone left the lid loose for a few days.
Essential Steps for Safe Storage
For daily use or long-term storage, start with a tight-fitting, clean plastic or glass container. Always label the jar with full details: substance name, hazard warnings, and the date it went onto the shelf. Good habits here save lives. I’ve seen accidents caused by reused containers with wrong labels or no warnings—one moment of confusion, and suddenly someone gets a chemical burn or a lungful of dust.
Keep cobalt chloride dihydrate away from high heat, sunlight, and moisture. Store it in a cool, dry cabinet designed for hazardous chemicals. A plain metal shelf in a humid storeroom will ruin it in weeks and may corrode the shelving as well. Ventilated cabinets help if there’s ever a spill or a broken jar. Separate it from acids and organic materials; this simple habit stops unexpected reactions that can create gases or other hazards in a cramped storeroom. Store it low, not above shoulder height, so there’s less risk of spilling the material onto a person’s face or upper body.
Training, Checks, and Culture
The best policies mean nothing if people on the ground don’t follow them. Regular reminders go a long way. In busy environments, it seems easiest to skip steps or rely on “how we’ve always done it.” I’ve watched labs thrive when senior staff take time for short training sessions, even five minutes during a shift. Quick audits—a supervisor checking the chemical shelf once a week—spot mistakes before something goes wrong. In places short on budget, even a checklist on the cabinet door keeps everyone honest.
Easy Fixes That Lower Big Risks
A few small investments pay off again and again. Transparent storage bins help workers spot leaks or dust right away. Silica gel packs inside the storage cabinet reduce moisture, a trick I picked up from a retired chemistry professor who never lost a single batch to humidity. For labs with tight quarters or lots of turnover, color-coded tape and custom labels stop confusion before it starts.
Protective gear matters—gloves, goggles, and good ventilation—each time, every time. Injuries from cobalt chloride dihydrate still happen in places where people get comfortable and just “grab a handful” to save time. That kind of shortcut has no place in responsible science, industry, or even an educational setting.
Moving Forward With Safety
Treating this chemical with respect isn’t only about following the rules. It’s about preventing avoidable accidents and saving money on ruined supplies. By keeping procedures simple and clear and investing in the right gear and habits, anyone who handles cobalt chloride dihydrate can keep themselves, their coworkers, and the wider community safe every day.
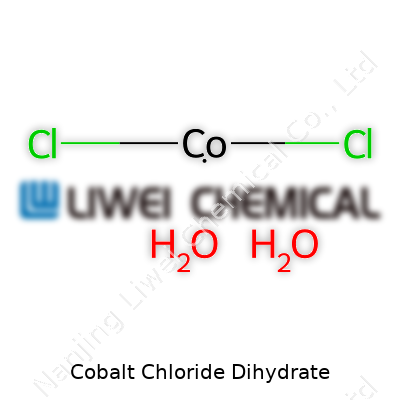
Understanding the Formula: CoCl2·2H2O
Cobalt chloride dihydrate rolls off the tongue kind of clunky, but the formula is pretty straightforward: CoCl2·2H2O. Reading it off a jar label never gives you the full story, though. Each cobalt atom pairs with two chlorine atoms and a couple of water molecules, locked into the crystalline structure. Those two little water molecules may not seem like a big deal, but they give this salt some unique properties in the lab. I’ve run into this compound most in high school and college chemistry. Its color signals hydration or dryness at a glance — the pink gives away its dihydrate status.
Practical Impact of Chemistry in Real Work
Anyone mixing up cobalt chloride dihydrate in the classroom or the lab probably notices changes fast. You get a gentle pink color with the dihydrate, clear as day, but let it dry out and the color flips to blue. The shift tells you moisture is gone. That makes this compound a go-to for humidity indicators. Toss a coated strip into a moisture-sensitive environment, and that subtle shift from pink to blue can save sensitive electronics, dry boxes, or packages from getting ruined by unexpected leaks in storage.
Industrial chemists rely on cues like these. Pharmaceutical processes require tightly controlled humidity, and cobalt chloride dihydrate lets teams know if those targets slip. Sometimes I get nostalgic for the days of tearing open silica packets, but in bulk settings that pink-to-blue color swing saves real money—catching a problem before it ruins a whole batch of product or storage supply.
Thinking about Safety
Chemicals can be fun to mix and play with, but not all of them are harmless. I’ve learned to handle cobalt compounds with respect. Exposure to cobalt chloride dihydrate can cause skin irritation, and breathing in dust isn’t healthy. Over time, trace cobalt builds up in the body, so labs stress gloves, dust masks, and proper cleanup. It’s a reminder: even fairly common chemicals pack a punch if taken lightly.
Looking up official guidance from sources like the CDC and OSHA helps keep workers safe. An MSDS sheet lays out the rules — no eating in the lab, use a fume hood, wash your hands. These measures sound basic but that’s what keeps trouble at bay. It doesn't add much time to a routine but makes a difference in health over months and years.
Alternatives and Environmental Considerations
Industries keep an eye out for less risky humidity indicators. Some companies have started switching to non-toxic alternatives where they can, especially for consumer uses. It’s not always possible — cobalt chloride offers high sensitivity and a clear visual cue — but greener options keep popping up. Research keeps pushing this further, trading out problematic compounds for safer and more sustainable replacements wherever they work reliably.
Looking Forward
That humble pink salt, with its formula CoCl2·2H2O, packs a surprising punch when it comes to its roles in science and industry. Staying informed about handling, health risks, and alternatives isn’t just a classroom exercise — it pays off in labs, production lines, and day-to-day operations. The formula is simple, the impact is widespread, and the chance for better, safer practices is always on the table.
The Reality in Labs and Classrooms
Cobalt chloride dihydrate draws a lot of attention partly because of its bright color, but also due to potential hazards. In university chemistry labs, this compound often turns up as a test for water presence—its color shift gives instant feedback. Most people see it and only think about its textbook uses, not its risks. The blue-to-pink transition seems harmless enough, but cobalt compounds don’t go easy on your health.
Cobalt salts carry real toxicity. The dust can irritate lungs and skin fairly quickly. I remember small spills in undergrad classes where a hasty reach would leave a faint pink on a palm. Aside from simple irritation, repeated or careless exposure may cause more serious concerns, including allergic reactions and long-term health effects if inhaled regularly. Cobalt chloride solutions also stain. That pink can follow you onto notebooks, keyboards, or even the inside lining of a pocket.
Practical Safety Steps
Preparation starts with gear. Gloves aren’t overkill, they're a necessity. Lab coats and closed shoes keep exposure down. Decent ventilation keeps airborne dust away from your face—open windows might seem enough, but a vent hood steps things up in a busy environment. After using cobalt chloride, no shortcut replaces decent soap and a full rinse up to the elbows. Keep it out of food areas, and never use the same glassware for cobalt chloride and anything else.
Spills tend to happen during weigh-outs or mixing. A scoop at room temperature stirs up more powder than you’d expect. Damping the powder gently with a little water before weighing cuts the risk of stray particles. Never sweep up dry powder; use wet towels or spill pads, and put all cleanup materials in a clearly labeled bag for later disposal.
Storing cobalt chloride right matters as much as using it safely. It absorbs water easily. Double-bagging and keeping bottles tightly sealed in a dry cabinet prevents messy drips and keeps accidental contact at bay.
Disposal and Responsibility
Tossing cobalt chloride in a sink or a regular trash bin isn’t just reckless, it creates environmental risks. Water systems can’t handle heavy metals, and cobalt compounds survive most sewage treatment. Local authorities treat cobalt waste like they do batteries or mercury thermometers. Hazardous waste collection programs often come around a few times a year, and schools or research labs always maintain a central chemical waste drum.
Label every container. Tidy handwriting and the date really help those doing waste pickups later. Pouring down the drain will only move the problem onto wildlife or downstream drinkers. In my own college town, a few improper disposals showed up years later in river quality tests—fixing that environmental slip took years and pulled funding from other community needs.
The real solution is preparation and respect for what cobalt chloride can do. Always store waste, tools, and unused reagent away from daily use zones. Ask for extra disposal containers if necessary. A short talk with building safety staff or a call to the local hazardous waste program gives peace of mind. There’s no glory in shortcuts—handling cobalt chloride safely means everyone stays healthy, and you keep the chemistry classroom or lab ready for the next group of learners.
| Names | |
| Preferred IUPAC name | Cobalt(II) chloride dihydrate |
| Other names |
Cobaltous chloride dihydrate
Cobalt(II) chloride dihydrate Cobalt dichloride dihydrate |
| Pronunciation | /ˈkoʊ.bɔːlt ˈklɔː.raɪd daɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 7791-13-1 |
| Beilstein Reference | 1701811 |
| ChEBI | CHEBI:31345 |
| ChEMBL | CHEMBL1201601 |
| ChemSpider | 69200 |
| DrugBank | DB11353 |
| ECHA InfoCard | ECHA InfoCard: 100.028.793 |
| EC Number | 231-589-4 |
| Gmelin Reference | 78573 |
| KEGG | C01341 |
| MeSH | D008802 |
| PubChem CID | 24584 |
| RTECS number | GF9825000 |
| UNII | Y4I4J14E58 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | 'CompTox Dashboard (EPA)': 'DTXSID8043883' |
| Properties | |
| Chemical formula | CoCl2·2H2O |
| Molar mass | 129.84 g/mol |
| Appearance | Blue to purple crystalline solid |
| Odor | Odorless |
| Density | 1.92 g/cm³ |
| Solubility in water | Deliquescent |
| log P | -2.219 |
| Acidity (pKa) | 6.4 |
| Basicity (pKb) | 7.8 |
| Magnetic susceptibility (χ) | +1600·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.716 |
| Dipole moment | 4.80 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 126.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -516.6 kJ/mol |
| Pharmacology | |
| ATC code | V03AB31 |
| Hazards | |
| Main hazards | Toxic if swallowed. Harmful if inhaled. Causes skin and serious eye irritation. May cause an allergic skin reaction. Suspected of causing cancer. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08, GHS09 |
| Pictograms | GHS07,GHS06 |
| Signal word | Warning |
| Hazard statements | H302, H317, H319, H334, H350, H410 |
| Precautionary statements | P201, P202, P261, P264, P270, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P314, P321, P333+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-0-Acide |
| Lethal dose or concentration | LD₅₀ Oral Rat: 766 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 766 mg/kg |
| NIOSH | T033 |
| PEL (Permissible) | PEL (Permissible Exposure Limit): 0.1 mg/m3 |
| REL (Recommended) | 0.02 mg/m³ |
| IDLH (Immediate danger) | **50 mg Co/m3** |
| Related compounds | |
| Related compounds |
Cobalt(II) chloride
Cobalt(II) chloride hexahydrate Cobalt(II) sulfate Cobalt(II) nitrate Nickel(II) chloride Copper(II) chloride |

