Cobalt Acetate: Behind the Chemistry, Risks, and Future
Historical Development
Interest in cobalt compounds goes back centuries, with dyed glass and ceramics revealing the blue pigment’s story. Cobalt acetate, compared to its oxide or sulfate cousins, arrived later along the timeline, gaining traction as both a lab staple and a tool for innovation. The advance of organic chemistry shined a light on cobalt’s catalytic quirks, pushing researchers to experiment. By the industrial revolution, workers noticed cobalt’s potential in drying alkyd resins and bit by bit, demand trickled from pigment-makers to battery researchers. This shift wasn’t driven by hype alone—cobalt acetate’s versatility slid easily into many scientific corners, guided by a mixture of chemical ingenuity and necessity.
Product Overview
Cobalt acetate, known in the industry as cobalt(II) acetate or cobalt diacetate, holds an important place on the chemist’s shelf. You’ll usually spot it as pink or purple crystalline solid, easy to weigh and dissolve. Labs count on its predictable behavior and stable storage, and the chemical doesn’t just gather dust—manufacturers turn to it for dyes, batteries, and synthetic reactions. What sets cobalt acetate apart isn’t just the cobalt; the acetate piece softens its chemistry, offering balance between reactivity and stability that unlocks a broader range of uses across sectors.
Physical & Chemical Properties
The compound has a distinct appearance. At room temperature, it forms a pink or reddish powder or sometimes little crystals, all thanks to the cobalt ions binding with water in a trihydrate form. Drop it in water, you’ll see it dissolve fast, ready for reactions. The formula, Co(CH3COO)2·4H2O, combines transition metal charm with straightforward organic acetate groups. Its melting point hovers near 140°C (dehydrates at that stage), and the color signals its place among hydrated cobalt salts. In air, it stands firm, not releasing fumes, keeping hazards manageable for storage and careful handling.
Technical Specifications & Labeling
Producers ship cobalt acetate with strict grades—technical, laboratory, and pure—each tailored to different end-uses. Purity percentages stay high, often 98% or above for lab and battery manufacture. Key specs include moisture content, presence of free acids, insolubles, and trace metal contaminants. Labels clearly display batch numbers, net weight, hazard symbols, producer contact, and shelf life, all to protect those who handle the product and maintain reproducibility in industrial batches. Any slip, and the trace metal tricks could upend a whole chemical process, so companies don’t cut corners here.
Preparation Method
The common laboratory method starts with cobalt carbonate or cobalt hydroxide, both easy enough to obtain. Add acetic acid, let it react in a beaker, and the solution yields cobalt acetate as acetic acid neutralizes the base. Industrially, the dance involves larger reactors and bumping up the temperature for efficiency. Sometimes, producers recover solid crystals by evaporating water or using a solvent like ethanol for controlled precipitation. No matter the setting, the approach avoids unnecessary byproducts, giving a cleaner product with little environmental aftertaste if handled properly. No magic here, just careful chemistry and years of optimization.
Chemical Reactions & Modifications
Cobalt acetate shines in reactions acting as a Lewis acid catalyst, especially in oxidation chemistry. It facilitates air oxidation of aromatic hydrocarbons and aldehydes—think big petrochemical refineries or specialty organic syntheses. In battery labs, it pops up during precursor synthesis for cobalt-rich cathode materials. Chemists tweak its reactivity further by swapping the acetate ligand or moving cobalt into mixed-valence states. Heating or reducing the compound with hydrogen strips away some acetate, yielding cobalt oxides or even metal—a pathway into magnetic and electronic materials. This connection fuels tweaks in everything from NMR-visible molecules to pigment formulations that withstand sunlight and chemical exposure.
Synonyms & Product Names
Many recognize cobalt acetate by trade names or chemical monikers: Cobalt(II) acetate tetrahydrate, cobaltous acetate, and EINECS 200-755-8. Some catalogues list it under CAS 6147-53-1 for the common tetrahydrate form, or as simply “Cobalt acetate” in battery supply chains. Globally, translation to Hindi, German, Chinese, and Russian reflects its industrial significance, and those in manufacturing circles often shorten the label in safety conversations for speed—just “Co-acetate” among plant workers. This tangle of names sometimes causes confusion, so keeping a careful eye on the actual formula and hydrate state matters when sourcing or using the compound.
Safety & Operational Standards
Workers know cobalt acetate as a hazardous chemical—respiratory irritant, potential carcinogen, and environmental pollutant. Inhalation, skin contact, or ingestion raise real risks, from acute irritation to chronic cobalt poisoning. Industrial processes require engineered ventilation, gloves, goggles, and tight inventory controls. Training programs drive home the need for careful labeling and proper storage away from food and flame. Regulatory bodies like OSHA and the European Chemicals Agency list cobalt acetate as a substance of concern, mandating exposure limits and spill protocols. Responsible handling helps maintain a safe workplace, putting health before speed or profit every step of the way.
Application Area
In manufacturing, cobalt acetate finds its way into drying agents for paints, varnishes, and inks, kickstarting polymer crosslinking to speed up hardening. The battery sector taps this compound while producing lithium-ion cathodes, where its even dispersion supports longevity and performance. Textile dyeing, pottery glazes, pet food additives, and vitamin B12 synthesis round out industrial use cases. Some biotech labs tap its reactivity for specialized protein studies or enzyme catalysis. Having handled cobalt salts in a university setting, I saw their impact firsthand—small amounts could steer whole student research projects, whether for pigment creation or for propulsion in organic synthesis.
Research & Development
Research teams explore cobalt acetate in new realms, from green catalysis to printed electronics and next-gen batteries. Lab notebooks fill with notes on ligand-tweaked analogues, seeking compounds that offer greater activity but less toxicity. Sustainable chemistry teams dig into recycling spent cobalt from batteries, eyeing closed-loop production. Universities test cobalt acetate’s ability to mimic metalloenzymes, chasing cheaper or more robust synthetic alternatives to natural biological catalysts. This arena isn’t static—researchers trial fresh acetate derivatives in nanomaterial synthesis or CO2 capture, seeking a cleaner tech footprint with improved performance in every cycle.
Toxicity Research
Scientists draw clear lines around cobalt’s dangers. Rodent studies reveal organ toxicity, reproductive harm, and possible links to cancer after chronic exposure. Inhalation of fine powders proves especially hazardous; inhaled cobalt sparks pulmonary issues, so workplace monitoring and personal protective equipment aren’t up for debate. Toxicology data informs regulators, steering permissible exposure limits and use-case restrictions. Environmental scientists examine the effect of cobalt acetate on aquatic life—runoff or spills don’t just fade away, but disrupt microbial and fish populations at surprisingly low concentrations. These findings steer industrial policies, funding new research into safer alternatives and better remediation strategies to curb long-term risks.
Future Prospects
The outlook for cobalt acetate tracks with shifts in energy storage and sustainability. Demand for rechargeable batteries lifts market interest even as companies scramble to reduce reliance on scarce or hazardous cobalt. Innovation leans on better recycling, smarter sourcing, and non-cobalt options for key uses. Regulatory tightening might shrink uses in consumer goods, while advances in green chemistry push scientists to design safer or more earth-friendly analogues. My own experience watching the battery field suggests cobalt acetate will remain important—but its footprint, both environmentally and in supply chains, will come under growing scrutiny. Those designing tomorrow's materials won’t just consider function—they’ll weigh long-term impacts, health, and planet alongside industry needs.
Cobalt Acetate and Its Unseen Impact
If you look past the long chemical name, cobalt acetate shows up in places most people never think about. I remember my college chemistry lab, where simple compounds like this sparked a lot more action than textbooks let on. Cobalt acetate looks like pale red crystals. It’s got a hand in several big-league industries. And sometimes what starts small at the molecular level steers much bigger trends in technology.
Catalysts in the Spotlight
Cobalt acetate steps up as a catalyst, especially in the production of terephthalic acid. This acid gives us PET plastic. Every time someone twists open a plastic bottle or rips open food packaging, there’s a good chance cobalt acetate played a role. The global demand for these plastics grows year after year, with millions of tons produced annually. Without the right catalyst, that production would stall or slow, trapping us in higher costs and resource shortages.
Paints, Pigments, and Everyday Color
Walk into any gallery or hardware store, and you’ll see an explosion of pigmented products. Cobalt acetate is part of what makes some blues, greens, and even dryers in varnishes possible. It doesn’t get the billboard credit, but it helps set the color and speed up drying so that paint jobs stay put instead of fading or smearing. Artists and contractors keep demand steady, often without even knowing it’s in the mix.
Batteries and High-Tech Hopes
The race to make better batteries puts cobalt compounds in the spotlight. Cobalt acetate finds its way into precursors for lithium-ion batteries. Phones, electric vehicles, and renewable energy storage all follow where this chemistry leads. The world’s thirst for reliable, long-lasting batteries keeps growing. Every jump in performance and stability relies on hard science, and cobalt acetate stands behind those gains.
Growing Concerns About Supply Chains
On the flip side, I’ve noticed growing chatter about where cobalt comes from. Much of the world’s cobalt is mined in challenging regions, and sometimes the supply chain runs into ethical questions or outright shortages. Risks range from price spikes to child labor in mining operations. The more the world leans on compounds like cobalt acetate, the more attention these issues get. Industries have started exploring recycling programs, new battery chemistries, and better sourcing standards. Groups like Amnesty International have pushed tech giants to map out their supply lines and improve transparency. The European Union and United States have launched strategic stockpiles and research into alternatives, but progress doesn’t happen overnight.
Innovation Around the Corner
Some start-ups and labs chase after ways to use less cobalt. Nickel, manganese, and iron-based chemistries make waves from time to time. Battery researchers keep scrounging for ways to cut costs and avoid supply crunches. At the same time, chemical engineers work on making recycling easier so fewer valuable metals get lost. Friends of mine in green tech talk about “urban mining”—extracting metals from discarded electronics—instead of drilling for fresh ore.
Why Cobalt Acetate Will Stick Around
If history tells us anything, industry doesn’t swap foundational materials overnight. Cobalt acetate’s role in essential manufacturing means it stays in the headlines any time tech, art, or environmental policy shifts. Supply concerns keep leaders up at night, while demand keeps climbing. The future will probably involve a mix of smarter recycling, smarter sourcing, and smarter chemistry. For now, cobalt acetate keeps the wheels spinning in some very big machines.
Understanding Cobalt Acetate Risks
Walking into a chemistry lab or an industrial workplace, signs with hazard symbols often catch your eye. Cobalt acetate shows up in industries like metal plating, ceramics, pigments, and some battery manufacturing. The label might look harmless, but the story behind it deserves attention. If you flip through safety datasheets, you’ll notice the health warnings attached to cobalt compounds. Cobalt ions, when introduced into the body, can throw off normal biological functions. That means everything from skin irritation to serious organ damage, depending on how you get exposed.
Everyday Exposure and Health Effects
I remember a coworker who ignored warnings about protective gloves. Over months of mixing chemical solutions, red patches and itchiness appeared on his hands. Turns out, cobalt acetate can aggravate existing allergies and trigger skin reactions. Inhaling dust from the powder or mist from solutions isn’t much safer. Industrial safety studies show that inhalation increases risks of lung problems. Scientists with the International Agency for Research on Cancer class cobalt compounds as possible carcinogens. Breathing in tiny airborne particles every day leaves workers with a higher chance for chronic lung diseases, scarring, and, in some cases, certain kinds of cancer.
Environmental Concerns
It’s not just the people using cobalt acetate who feel the impact. Factories sometimes mishandle hazardous waste, letting chemicals reach waterways. Once it arrives in streams and rivers, aquatic life faces serious harm. Fish exposed to cobalt build up toxins in their tissue, which can move up the food chain. Farms downstream can end up with soil loaded with heavy metals. As someone who grew up fishing in local ponds, I’ve seen the effects over time — once-busy spots clear out as smaller species vanish.
Why Cobalt Acetate Still Gets Used
You might ask: given the risks, why do plants and labs keep ordering cobalt acetate? The answer ties back to its powerful chemical abilities. In batteries, it helps manage charge and discharge cycles. In ceramics and paints, it gives products lasting color and durability. Cutting it out completely isn’t simple, since few alternatives can match its performance. But with every benefit comes responsibility. Choosing safety can’t be an afterthought once people get sick or ecosystems decline.
Sensible Solutions for Handling Hazards
Protecting people and the environment starts at the source. Clear labeling and proper training make a difference. In my years working with hazardous substances, face masks and gloves became everyday gear. Not every company sets high standards, but the best ones take extra steps: ventilation, dust collection, medical checkups, and backup plans for accidental spills. On a community level, environmental watchdogs and local activists put pressure on plants to track their chemical waste. Regulations from agencies like the EPA or OSHA add extra teeth, with penalties for leaks and unsafe practices. Science won’t stop searching for less toxic alternatives, either. Some research explores ways to recycle or neutralize spent cobalt materials from batteries and paints to cut down on the demand for fresh supply.
Navigating the Path Forward
Cobalt acetate carries risks that hit workers, communities, and ecosystems. Safety never comes from wishful thinking; it grows through real steps—education, personal protection, smart engineering, and oversight. Staying informed and demanding transparency allows safer practices to win out over short-term profit or convenience. Life and work should come home safe at the end of the day, not weighed down by silent chemicals with hidden costs.
The Formula and Its Roots
Chemistry may put off a lot of people. All those letters and numbers throw us back to high school labs—with the strong smell of vinegar in the air and blue crystals bubbling in a flask. But taking the time to look at a substance and genuinely think about what its chemical makeup means pays off later. Cobalt acetate, for instance, has the molecular formula Co(CH3COO)2. It sounds straightforward: one cobalt atom sits at the center, and it’s surrounded by two acetate groups. These acetates come from acetic acid, the stuff that gives vinegar its punch. That basic little combination ends up shaping how cobalt acetate works in batteries, pigments, and even as a catalyst. You wouldn’t make the connection just looking at the formula, but the real-world impact is much broader than most folks expect.
Daily World of Chemistry
We brush against chemistry all day long, most of us unknowingly. Take batteries—especially old-school rechargeables. Cobalt compounds are behind the color on a ceramic bowl, the charge in a battery, the coloring in glass. That stuff on the label may say “cobalt acetate,” but unless you’ve poked at the formula, it’s not clear what that means for the world at large. The formula tells a clear story: carbon, hydrogen, and oxygen from acetate teams up with cobalt. Those four simple elements build something that can interact with metals, bind dyes, or speed up a chemical reaction in a factory. This formula is more than a string of symbols. It’s a blueprint for how the substance works—why it clings to certain materials, or dissolves in water, or latches onto other atoms in a chemical dance.
Risks and Responsibility
Ask anybody who’s spent time around lab benches or worked in industry—they’ll tell you handling cobalt compounds requires a dose of respect. The formula, by showing exactly what’s in the compound, helps highlight that risk. Cobalt itself can be hazardous: inhaling its dust or fumes, frequent skin contact, even casual exposure, brings real health concerns. Acetate can irritate, too. Chemical safety data is not just legal fine print. It exists because chemists looked at the formula, ran the tests, and found real risks. It serves as a reminder: using something as common as cobalt acetate in pigments and batteries carries health and environmental baggage.
The Path Forward
As someone who has watched the rise of lithium-ion batteries and greener tech, I recognize a shift in the way industries pay attention to what goes into their products. Nobody wants heavy metals leaching into the environment, or unreliable products in a child’s hands. The push for more sustainable chemistry pushes folks to re-examine molecular formulas, and rethink what’s really required to get the job done. Labs experiment with alternative catalysts, and new dyes that avoid cobalt altogether. While the basic formula says, “Here’s what you’re dealing with,” current practice asks, “Is there something safer out there?”
Why It Matters
Anyone who’s peeled a label off a cleaning product or tried to decode an ingredient list has felt that frustration: What do all those numbers and letters mean? The truth is, knowing the molecular formula behind substances like cobalt acetate arms us to make better decisions—at the industrial level, in government policy, and at home. Anyone can search facts and see that Co(CH3COO)2 means more than meets the eye. If we want a cleaner future, understanding these formulas needs to move beyond the research lab and into daily life, so choices become responsible and consequences don’t catch us by surprise.
Understanding the Risks and Responsibilities
Cobalt acetate works as a common ingredient in laboratories and industrial environments, showing up in plenty of chemical processes and battery manufacturing. Most folks who deal with it recognize its purple-pink hue and powdery look, but fewer pause to think about its risks. Anyone who has spent time around chemicals knows that even the most ordinary compounds demand some respect. Problems rarely pop up out of nowhere, but small lapses—the lid not tightened, the container sticking around on the wrong shelf—add up until real hazards start to show.
Health and Safety Take Center Stage
Exposure to cobalt acetate has health implications nobody should overlook. Inhaling its dust, or letting it touch skin, can cause irritation or even worse effects after long exposure. There's a reason workplace regulations, like those from OSHA and NIOSH, set thresholds for how much can float around in the air. Years ago, I worked in a warehouse where improper storage meant one leaky bag could coat a storage room with a fine, toxic dust. Simple habits—wearing gloves, using a fume hood, sealing packaging—spare everyone a lot of trouble and medical bills.
Keeping Cobalt Acetate Secure
Cobalt acetate belongs in a cool, dry spot—preferably, locked away from the bustle of main storage. Moisture invites lumps and chemical reactions, and nobody likes seeing their chemical investment spoiled. It reacts with acids and strong oxidizers, so separating it from incompatible materials should always be a priority. I learned early in my career that storing chemicals alphabetically serves a library, not a lab. Grouping by compatibility trims the odds of a dangerous mix-up. Physical barriers matter more than clever lists stuck to the wall.
Use of labeled, corrosion-resistant containers forms the backbone of safety. Metal shelves often work well, but plastic bins bring a safety net when paired with secondary containment trays. Think of double-bagging groceries, but with much higher stakes. Accidents rarely happen during big experiments—they show up when someone knocks a bottle over, so secondary containment helps catch those mishaps before they turn into emergencies.
Reducing Environmental Impact
A lot of labs ignore the role of long-term storage on the environment. Leaks and spills sneak into water systems or soil. Tracing pollution back to a single bottle of cobalt acetate rarely happens, but local communities feel the effects. Spill control kits and regular inspections should feel routine, not special. Facilities with strict waste management keep both staff and neighborhoods safer. Small changes—like replacing aging containers and checking seals—pay off by preventing disasters.
Taking Ownership at Every Level
Transparency forms the foundation for any trustworthy workplace. Training stays useful when folks actually understand why rules exist. Safety datasheets, regular drills, and visible warning signs build habits that stick. I remember one job where the oldest tech in the room always pointed out storage mistakes, guiding the team by sharing horror stories instead of just pointing to paperwork. Peer-to-peer support kept us on track and, indirectly, kept everyone healthy.
Cobalt acetate plays a growing role in manufacturing and research. Safe, sensible storage lets organizations pursue innovation without sacrificing health, reputation, or environmental quality. Taking shortcuts always looks easy until a costly cleanup or regulatory fine comes calling. Real expertise means knowing more than facts—it means following through, day after day, on the habits that keep people safe.
A Closer Look at Its Appearance and Structure
Cobalt acetate usually turns up in laboratories as deep red, chunky crystals. Hold a handful under sunlight and the color grabs your attention. The crystals gleam with a reddish tint that stands out even beside other common acetate compounds. This shade comes from cobalt itself, which tends to color salts in distinctive ways, popping up in artist supplies, chemistry experiments, and industry.
Solubility and Practical Use
Drop cobalt acetate into water and the crystals break apart fast. That high solubility makes it simple to use in solutions. Chemists and engineers often count on this trait: it dissolves easily so there’s no need for strong acids or heavy shaking. You notice the characteristic red or pink color in the solution, a sign there’s still cobalt in action and not just another salt. Its ability to dissolve rapidly in ethanol broadens its use beyond water-based mixes, allowing more options in process design and material production.
Handling Stability and Storage Points
Humidity becomes the enemy for this compound. Cobalt acetate absorbs moisture from the air, sometimes forming a clump if left out exposed. That “deliquescent” behavior leads to a sticky mess on the lab shelf if storage rules slip. Keeping it in a tightly sealed container slows down this problem.
Heating triggers changes. At higher temperatures, the substance releases water in steps, losing its hydrated part and eventually breaking down. If you’ve ever left a sample near a heat source by mistake, you’ll notice it grows powdery, and the color shifts a bit. Controlling the storage space, especially avoiding direct sun and dampness, keeps the crystals in their original form longer and cuts down on waste.
Density, Weight, and Reactions
This acetate includes cobalt, so the crystals feel heavier compared to typical table salt or sugar. Its density usually lands just under 1.7 grams per cubic centimeter for the hydrated type. That extra weight might not show up much in a scoop, but in large batches it affects shipping, storage, and safety planning.
Another aspect stems from cobalt’s reactivity. Cobalt acetate doesn’t sit idle in a jar. In air, it slowly reacts if mixed or spilled; exposure leads to oxidation, sometimes shifting shades and even affecting the end use in chemical processes or pigments.
Health and Environmental Notes
Many people overlook the safety side. Cobalt can irritate skin and lungs if handled poorly, especially during weighing or mixing. Airborne powder poses a risk, which makes a dust mask handy, along with gloves. Cobalt acetate counts as hazardous waste in several countries, so disposal means following environmental regulations closely. This prevents metal ions from building up in local waterways, a real concern around manufacturing hubs.
Role and Potential Solutions
In industry, its physical properties steer where and how it gets used—from catalysts to material coloring. Manufacturers often look for ways to keep moisture out and manage dust. Vacuum-sealed packaging helps. So does training workers, cutting down exposure and accidents on the shop floor. There’s ongoing research in safer formulations and substitutes, but cobalt acetate holds its position thanks to its tailored mix of solubility, stability, and color. Smart handling and updated safety gear address most routine risks.
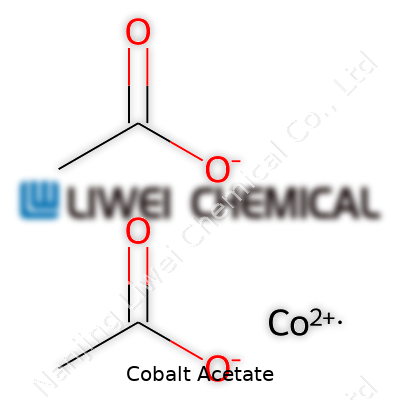
| Names | |
| Preferred IUPAC name | cobalt(2+) acetate |
| Other names |
Cobalt(II) acetate
Acetic acid, cobalt(2+) salt Cobalt diacetate Cobaltous acetate |
| Pronunciation | /ˈkoʊ.bælt ˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 71-48-7 |
| Beilstein Reference | 3589538 |
| ChEBI | CHEBI:62973 |
| ChEMBL | CHEMBL1233892 |
| ChemSpider | 54816 |
| DrugBank | DB11335 |
| ECHA InfoCard | 100.006.721 |
| EC Number | 200-755-8 |
| Gmelin Reference | 5592 |
| KEGG | C01414 |
| MeSH | D003054 |
| PubChem CID | 3033972 |
| RTECS number | AG7520000 |
| UNII | GX18M39NO6 |
| UN number | UN3283 |
| Properties | |
| Chemical formula | Co(C2H3O2)2 |
| Molar mass | 177.07 g/mol |
| Appearance | Blue crystalline solid |
| Odor | vinegar-like |
| Density | 1.705 g/cm³ |
| Solubility in water | Very soluble |
| log P | -1.03 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa 4.76 |
| Basicity (pKb) | 9.7 |
| Magnetic susceptibility (χ) | +1380e-6 |
| Refractive index (nD) | 1.542 |
| Dipole moment | 4.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -819.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1615.7 kJ/mol |
| Pharmacology | |
| ATC code | V03AB56 |
| Hazards | |
| Main hazards | Toxic if swallowed, inhaled or in contact with skin; may cause cancer; suspected of causing genetic defects; may cause allergy or asthma symptoms or breathing difficulties if inhaled; causes skin and serious eye irritation. |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H317, H319, H334, H341, H350, H360, H410 |
| Precautionary statements | P210, P261, P273, P280, P302+P352, P304+P340, P308+P313, P314, P321, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Autoignition temperature | 485°C |
| Lethal dose or concentration | LD50 oral rat 708 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 6,821 mg/kg |
| NIOSH | MI14000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 1 mg/m³ |
| IDLH (Immediate danger) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
Cobalt(II) carbonate
Cobalt(II) oxide Cobalt(II) chloride Manganese(II) acetate |

